Recently, Prof. Jiajing's group from our college published a research article titled "Sulfonium-based precise alkyl transposition reactions" in Science Advances (Sci. Adv., 2023, 9, eadi1370). (Fig. 1).
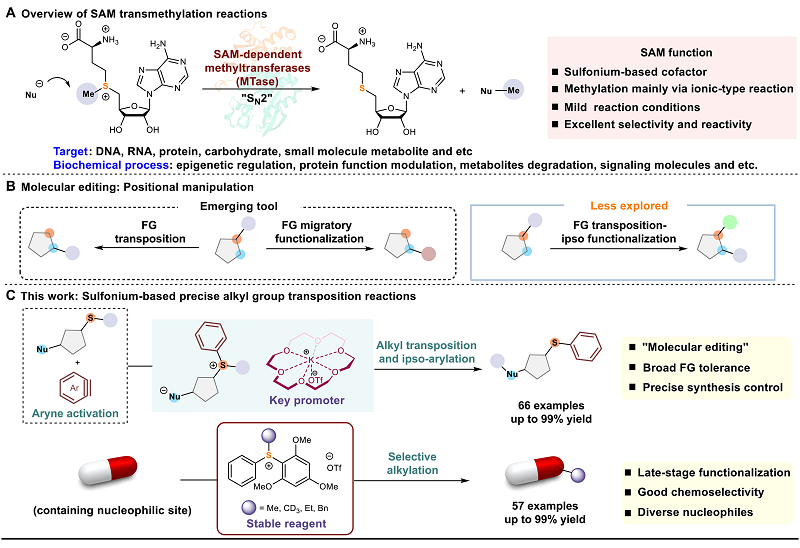
Fig. 1 Summary of the research
S-adenosyl-L-methionine (SAM) is an important cofactor containing a sulfonium skeleton. In living organisms, SAM is formed via adenosine triphosphate (ATP) and methionine in the presence of methionine adenosyl transferase, and it plays the role of methyl donor in the complex group transfer process in living systems, which can participate in the methylation reactions of DNA, RNA, proteins, carbohydrates, and small molecule metabolites. Inspired by the synthetic functions and applications of sulfonium in nature, the team cleverly exploited the properties of sulfonium intermediates to successfully develop an aryne-induced alkyl migration/arylation reaction due to the situation. The sulfur-containing amphiphile intermediate was constructed by using the electrophilic activation strategy of aryne, and the sulfonium generated in situ via proton transfer can be used as an electrophilic alkylation reagent to react with another nucleophilic site of the substrate to realize alkyl migration and in situ arylation, with the net result of migration of functional groups. The reaction can occur smoothly on structurally complex molecular skeleton with a wide range of functional group tolerance, demonstrating its potential application as a molecular editing tool. Quantum studies show that the KF/18-C-6 complex demonstrates a unique promoter in this reaction. The team also designed and developed a series of stable sulfonium reagents that can be selectively alkylated with multiple classes of nucleophilic substrates under mild conditions. Both types of reactions can be used for late-stage modification of drug molecules, further emphasizing the advantages of precision synthesis.
S-adenosyl-L-methionine (SAM) is a vital cofactor in living organisms, characterized by a sulfonium skeleton. It is synthesized from adenosine triphosphate (ATP) and methionine in the presence of methionine adenosyl transferase. SAM serves as a methyl donor in various group transfer processes, participating in the methylation of DNA, RNA, proteins, carbohydrates, and small molecule metabolites. Drawing inspiration from the synthetic roles and applications of sulfonium in nature, Prof. Jiajing's team skillfully leveraged the properties of sulfonium intermediates to innovate an aryne-induced alkyl migration/ipso-arylation reaction. The nucleophilic addition of substrate to aryne generated the zwitterionic intermediate, which deprotonated pre-nucleophiles to form key sulfonium salt intermediates, facilitating the alkyl-transfer reaction. The protocol proceeds smoothly on structurally complex molecules, exhibiting broad functional group tolerance, which should display great potential as a molecular editing tool. Quantum studies reveal that the KF/18-C-6 complex plays a unique role as a promoter in this reaction. The team has also developed a series of stable sulfonium reagents, capable of alkylating various nucleophilic substrates under mild conditions with good selectivity. Both methods hold promise for late-stage modifications in drug development, showcasing the benefits of sulfonium chemistry toward precision synthesis.
Prof. Jiajing Tan thanks for the funding support from the National Natural Science Foundation of China (22271010), and BUCT.
