Recently, Prof. Xiang Guolei and Wang Leyu published a research paper in Nature Communications entitled Interfacial compatibility critically controls Ru/TiO2 metal-support interaction modes in CO2 hydrogenation (Nat. Commun. 13, 327 (2022), https://doi.org/10.1038/s41467-021-27910-4), exploring the interface design principle of CO2 hydrogenation catalysts.
Heterogeneous catalysis is one of the core technologies in chemical industry, and is widely used in the production of bulk and fine chemicals, environmental protection, and energy conversion. The catalytic performances (activity, selectivity, stability) are not only influenced by the composition, morphology, and size of metal nanoparticles, but also by the support material. Therefore, metal-support interaction (MSI) is a fundamental issue in catalytic science. Numerous studies have shown that the interaction between metal and support can be used to control the activity and selectivity of catalysts through strong metal-support interaction (SMSI), interface charge transfer, and the formation of boundary layers. However, some key scientific questions remain unclear, such as the structural factors crucial for controlling different MSI modes, the regularity of SMSI, and the nature of structure-performance relationships in catalysis.
This study reveals that the nature of the metal-support interfaces play a crucial role in MSI modes. The metal-support interface contact is the basis for all MSI phenomena, so understanding this interaction at the atomic level is crucial for revealing key scientific issues in the field of heterogeneous catalysis. The study selected Ru/TiO2 as a model system and altered the Ru-TiO2 interface compatibility by annealing. Although R-TiO2 (rutile) and A-TiO2 (anatase) have the same chemical composition, their crystal structures differ. RuO2 and R-TiO2 have the same crystal structure, allowing for the construction of different contact interfaces on the two supports. By comparing the catalytic performance of directly reduced and annealed-reduced catalysts in CO2 methanation, they found that annealing led to two completely opposite catalytic phenomena on R-TiO2 and A-TiO2 supports. The catalytic activity significantly increased on R-TiO2 support, while it sharply decreased on A-TiO2 support, accompanied by a change in selectivity. They reveal that air plays an important role during the annealing process.
The first authors of this article are our master's student Zhou Jun and Prof. Gao Zhe from the Shanxi Coal Chemical Institute. Xiang Guolei and Wang Leyu are the corresponding authors. This work was supported by the National Natural Science Foundation and the National Key Research and Development Program.
|
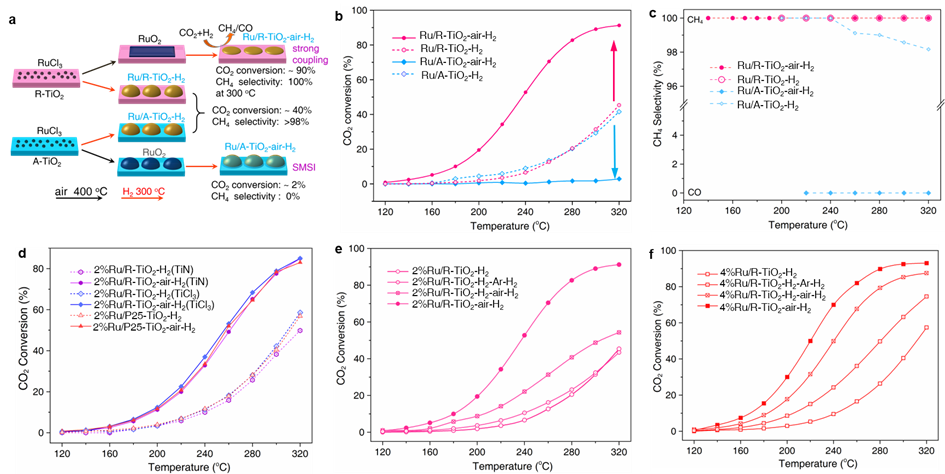
Fig. 1. Opposite catalytic performances of Ru/TiO2 supported on rutile (R-TiO2) and anatase (A-TiO2) for CO2 hydrogenation. (a) Summary scheme of varied activity and selectivity by direct H2 reduction and after annealing in air. Ru/TiO2-H2 refers to directly reduced catalysts by H2, while Ru/TiO2-air-H2 refers to catalysts by annealing in air at 400 oC and further reduction by H2. (b) Temperature-dependent CO2 conversions. (c) Temperature-dependent CH4 selectivity. (d) Temperature-dependent CO2 conversions of 2%Ru/R-TiO2 catalysts on other R-TiO2 supports that were prepared from TiN and TiCl3, and P25 TiO2. Temperature-dependent CO2 conversions by annealing (e) 2%Ru/R-TiO2-H2 and (f) 4%Ru/R-TiO2-H2 catalysts in Ar and air. Ru/R-TiO2-H2-air-H2 means the catalyst was first reduced by H2 at 300 oC, then annealed in air at 400 oC, and reduced with H2 at 300 oC again.
|
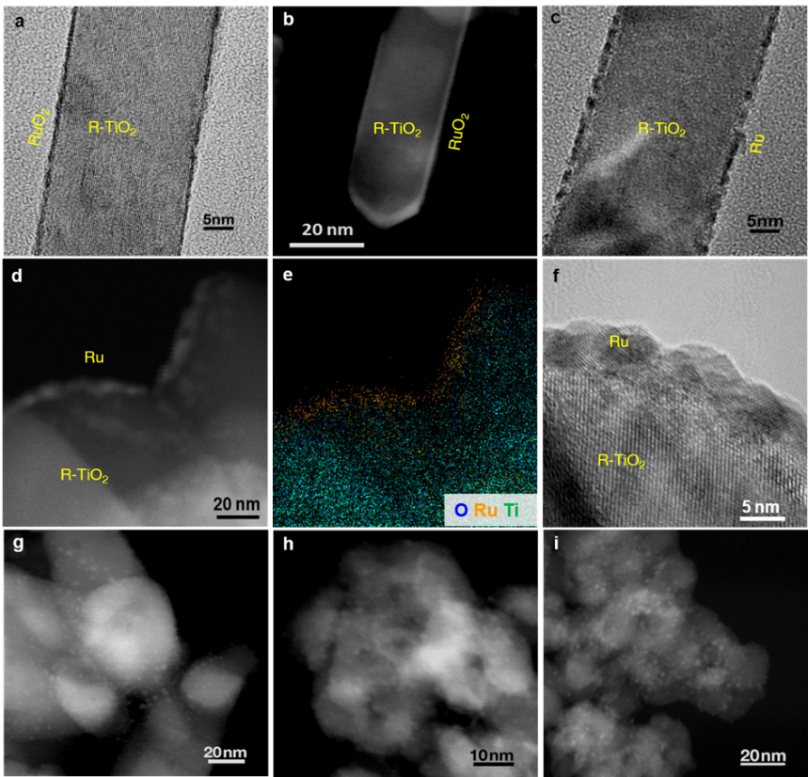
Fig. 2. Structure characterizations of Ru/TiO2 catalysts. (a) TEM and (b) STEM images of RuO2 overlayers on R-TiO2 nanorods. (c) Ru/R-TiO2 reduced from RuO2/R-TiO2 by H2. (d) STEM, (e) elemental mapping, and (f) TEM images of Ru/R-TiO2-air-H2 after CO2 hydrogenation. STEM images of (g) Ru/R-TiO2-H2, (h) Ru/A-TiO2-air-H2, and (i) Ru/A-TiO2-H2 after reactions.
|
Fig. 3. State characterizations of Ru species on TiO2 supports. (a) H2-TPR of RuO2/A-TiO2 and RuO2/R-TiO2. (b) XRD patterns and (c) XPS spectra of Ru/TiO2 catalysts.
|
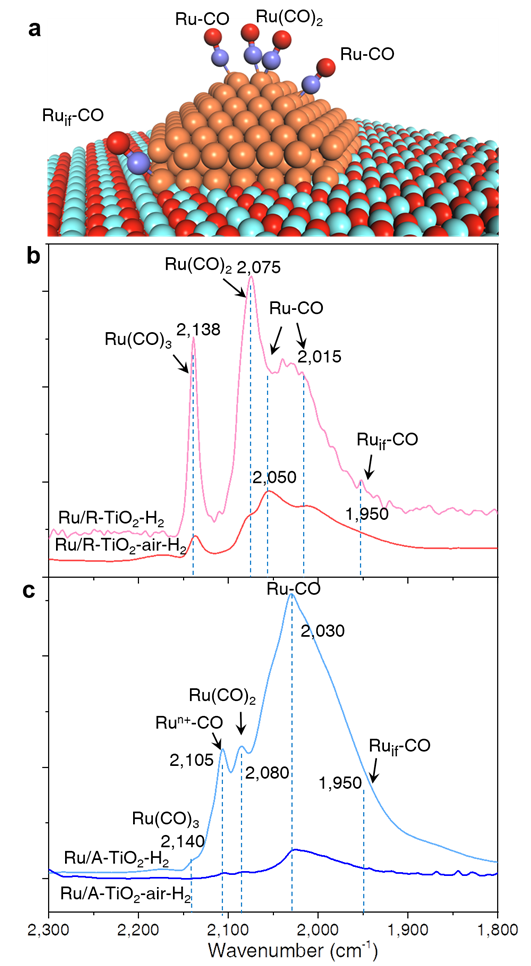
Fig. 4. Surface atomic states of Ru NPs probed with CO-DRIFTS at 25 oC. (a) Adsorption configurations of CO on Ru/TiO2 catalysts. (b, c) CO-DRIFTS of Ru/TiO2 catalysts.
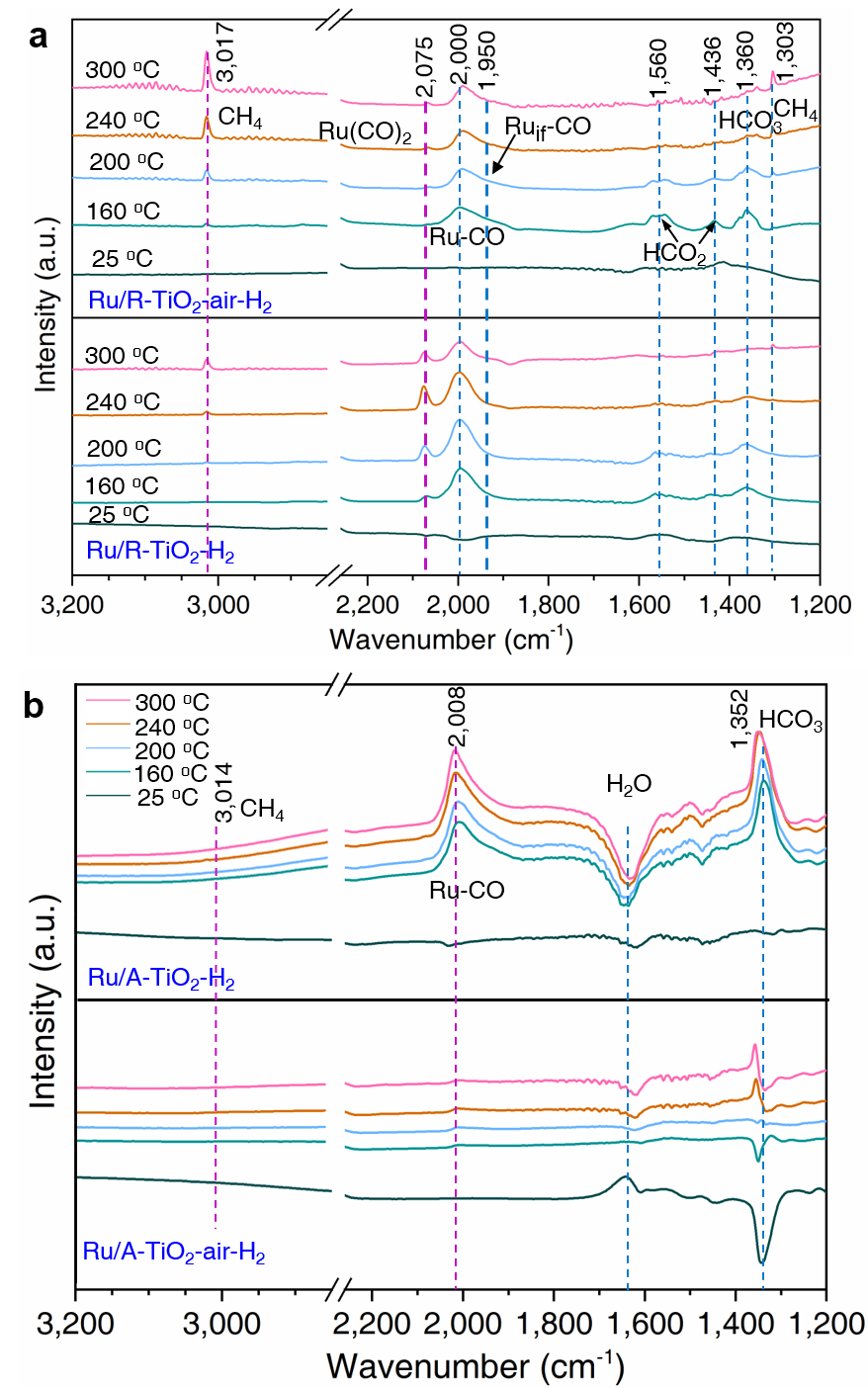 |
Fig. 5. Revealing reaction mechanisms of CO2 hydrogenation on Ru/TiO2 catalysts with operando FTIR from 25 oC to 300 oC. (a) Reaction mechanisms on Ru/R-TiO2-H2 and Ru/R-TiO2-air-H2. (b) Reaction mechanisms on Ru/A-TiO2-H2 and Ru/A-TiO2-air-H2.