
In the recent years, polymer-based room-temperature phosphorescent materials have attracted wide attention because of their low cost, easy processing ability and good biocompatibility. Noteworthy that the simultaneous requirements of robust mechanical properties and decent phosphorescence performances are demanded to prepare flexible RTP materials for the practical applications.
The addition of inorganic components to polymer-based RTP materials provides a rigid environment that inhibits nonradiative transitions of chromophores and enhances the mechanical properties of polymermatrix. However, the current construction of composite materials is generally based on the hydrogen bonding and electrostatic interaction between polymers and inorganic components, suffering from the weakened interaction by the moisture in the air and thus the quenched phosphorescence or mechanical properties.
To solve this problem, Rui Tian and Chao Lu in the College of Chemistry, Beijing University of Chemical Technology, have introduced the facile B‒O click reaction to prepare room-temperature phosphorescent materials with high mechanical strength based on the covalent bond between the inorganic layered double hydroxide (LDHs) with rich hydroxyl groups, chromophores (BPBA) containing boric acid groups, and polyvinyl alcohol (PVA) matrix. The as-prepared composites exhibited an ultra-long lifetime of 1.45 s and decent mechanical strength of 97.9 MPa, which could find great potential in the applications as anti-counterfeiting devices, security labels and organic light-emitting diodes.
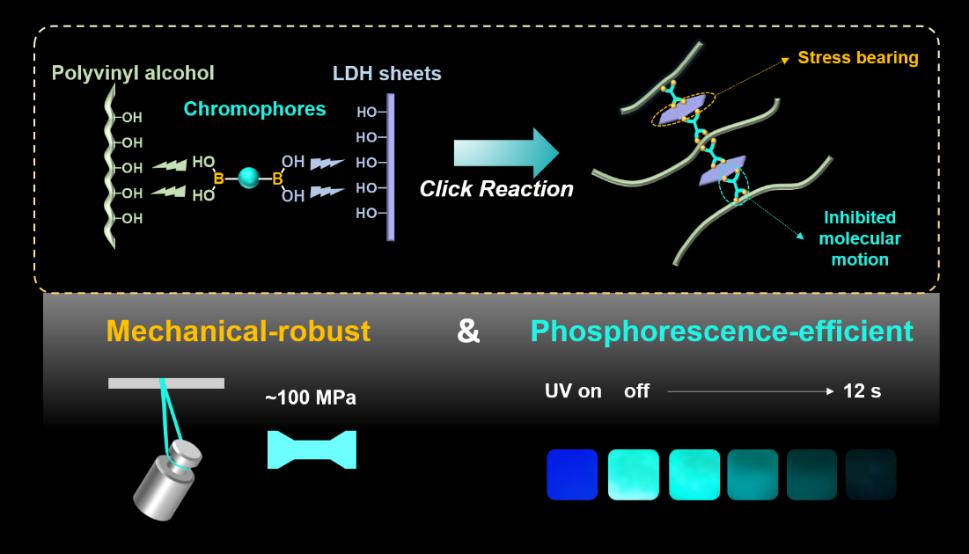
Fig. 1 Schematic representation for the mechanical-robust room temperature phosphorescence materials constructed by the covalent bonds.
The prepared LDHs-BPBA-PVA exhibited cyan phosphorescence emission at 495 nm, with a phosphorescent lifetime of up to 1.45 s, which could be traced to more than 12 s by the naked eyes (Fig. 2).The phosphorescent properties of LDHs-BPBA-PVA composites were greatly enhanced. Such an enhancement could be ascribed to the multiple B‒O covalent bonds between PVA, BPBA and LDHs, which effectively inhibited the non-radiative decay of excited triplet excitons and enhanced the intersystem crossing process in the composite films. The function of B‒O covalent bonds was validated by adjusting the quantities of hydroxyl groups in PVA and LDHs, respectively. With the decrease of hydroxyl sites, the phosphorescence performance of LDHs-BPBA-PVA composite film decreased significantly, and FT‒IR spectroscopy confirmed that vibration peaks of B‒O stretching gradually decreased.
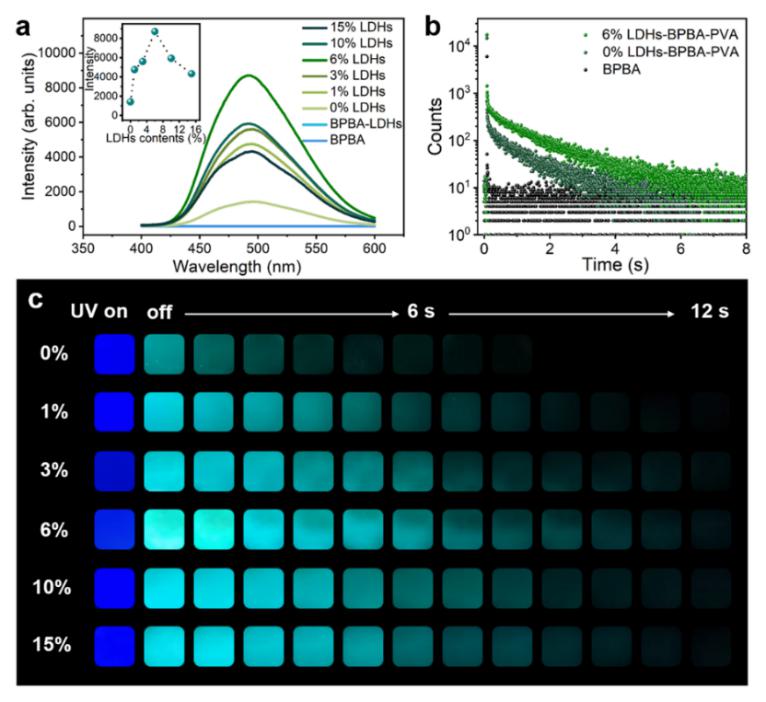
Fig. 2 RTP emission spectra, lifetime measurements and photographs of x%LDHs-BPBA-PVA films.
The mechanical properties of the composite films were further studied (Fig. 3).Notably, the tensile strength of 6% LDHs-BPBA-PVA reached 97.9 MPa after the covalent bonding, which was 2.17 times that of PVA. The significantly promoted mechanical properties indicated that BPBA played a key role as a cross-linking agent to reinforce the interfacial interaction between organic PVA and inorganic LDHs phases. Such a mechanical-reliable, phosphorescent-robust and scalable film could find great potential in flexible 3D objects with repeatable folding and crimping properties in a large scale.
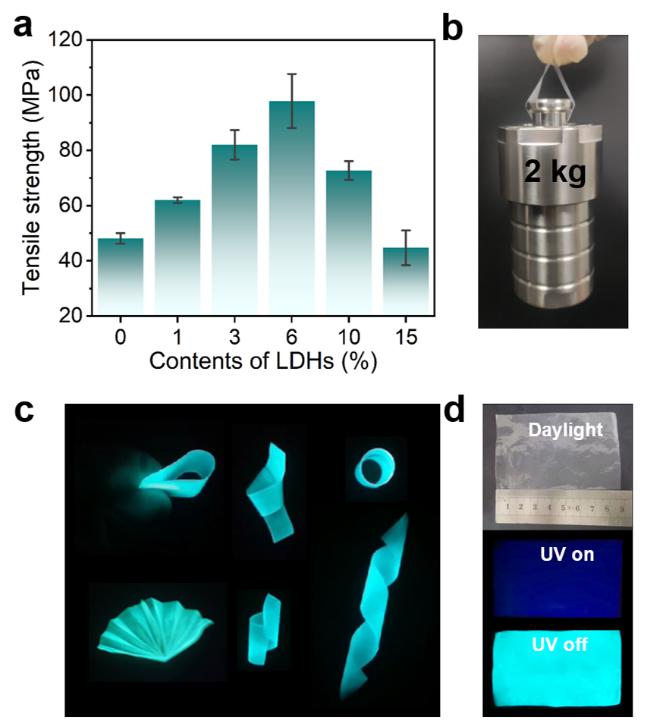
Fig. 3Mechanical properties of LDHs-BPBA-PVA composite films.
In summary, a decent combination of the phosphorescence and mechanical performances has been achieved for the polymeric composites based on the file and tunable B–O click reaction. Decent scalability, flexibility, stretchability and sensitive phosphorescence responses towards mechanical deformation have also been realized. It is believed that the proposed strategy could be further applied for the design and construction of functional composites with advanced performances.
Information for the manuscript:
Title: Design of mechanical-robust phosphorescence materials through covalent click reaction
https://www.nature.com/articles/s41467-023-40451-2
