Recently, Professor Junqing Pan Group at college of chemistry published a research paper entitled “Ultra-Fast Recyclable and Value-Added Desulfation Method for Spent Lead Paste via Dual Intensification Processes” in Advanced Science (Adv. Sci. 2023, 2304863).
The pursuit of a new low-cost clean pre-desulfation technology of lead paste is very important in the pyrometallurgy and hydrometallurgy for recovering recycled lead resources.However, traditional reactors have low space-time yield and desulfation rate, resulting in high energy consumption and SO2 emissions in the industrial desulfation processes. The major reasons for the low desulfation rate are the following problems: (1) Most stirred reactors own the limited space and low shear force, leading to a low conversion rate and increasing the energy consumption due to the unreacted raw materials to be covered by the generated product coating layer. (2) Most stirred reactors have long reaction time and low space-time yield due to low kinetic rate. (3) Most of the stirred reactors possess a discontinuous reaction process, resulting in the low production efficiency and the inconsistent products of different batches.
In this work, we propose an ultra-fast recyclable and value-added desulfation method for spent lead paste via dual intensification processes in which two newly introduced RLFRs for the enhancement of the desulfation process of spent lead paste and sulfation process of lime and mother liquor (Fig. 1). In this case, the RLFR is proposed as a new intensification device to rapidly collide raw materials efficiently by instantaneous solid-liquid surface renewal with a greatly increased mass transfer rate to break through the diffusion-controlled chemical reaction process. Compared with the low reaction efficiency, long reaction time, and discontinuous reaction caused by the reaction products coated on the unreacted raw materials in the traditional reactor, (1) the RLFR possesses the tremendous shear force generated by the rotor-stator system, which can make the prepared materials have the characteristics of small average particle size and narrow particle size distribution, greatly enhancing the microscopic mixing and mass transfer process. (2) The RLFR with the small confinement space and high rotation speed, makes raw material and liquid rapidly pass through the gap between the high-speed rotor and stator under the action of extrusion, which causes the continuously exposed new surfaces to contact with the desulfation agent, thus greatly shortening the desulfation time and improving the reaction efficiency. Thus, the new device instantly generates large grinding force and enough transformation chance among material particles, leading to the forced renewal material surface and the instantaneous accomplishment of reaction.
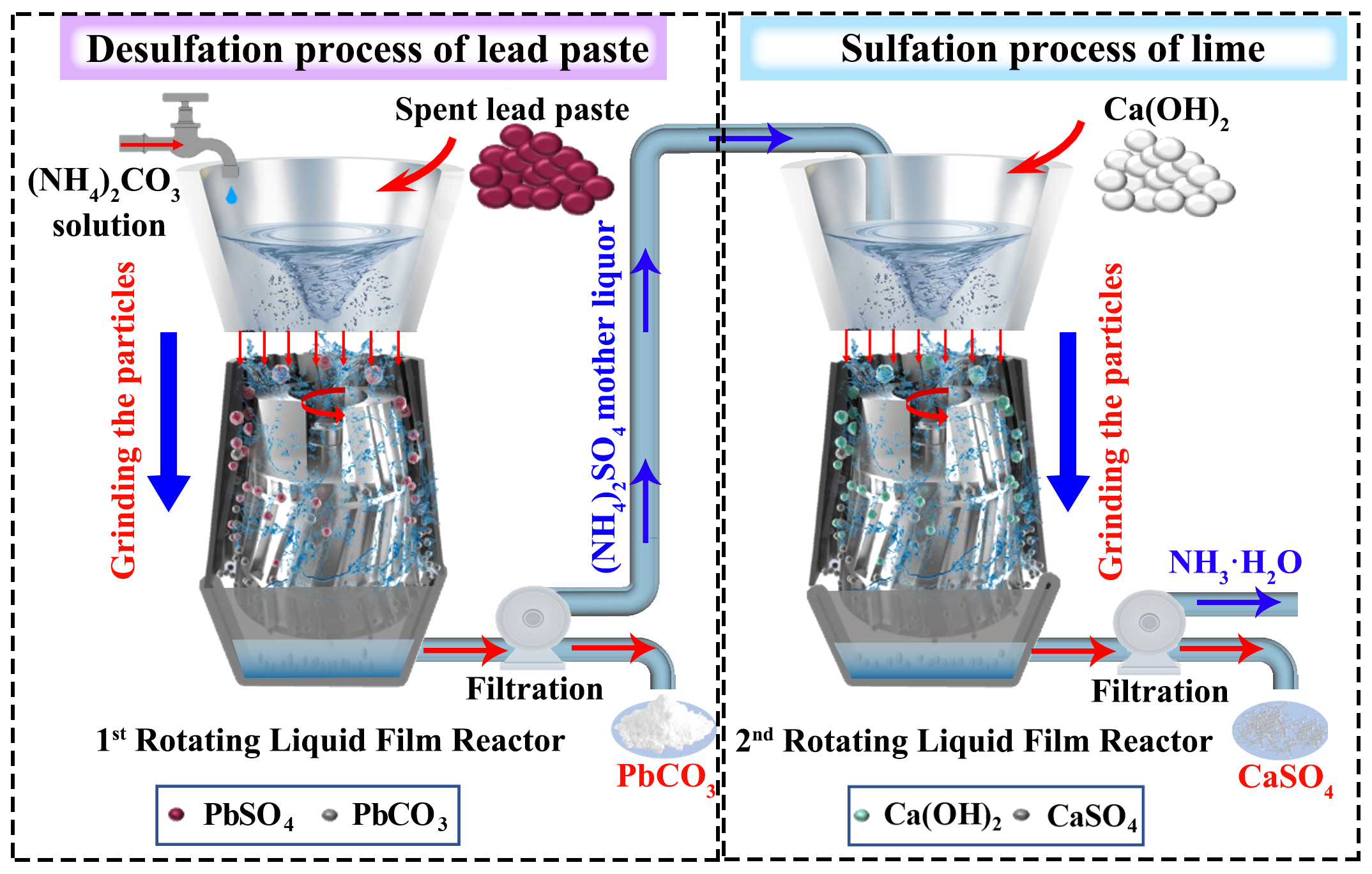
Fig. 1. Schematic diagram of a new ultra-rapid desulfation process of spent lead paste and sulfation process of lime via newly designed rotating liquid film reactors (RLFRs).
In this paper, Fig. 2 shows the desulfation process of spent lead paste and (NH4)2CO3 and the sulfidation process of (NH4)2SO4 mother liquor and Ca(OH)2 by the intensification process of RLFR, which improves the inherent shortcomings of traditional reactors, revealing the versatility for different types of reactions. The new RLFR intensification process achieves that the desulfation rate and conversion rate are 99.7% and 98.6% in 10 and 30 s, far higher than the 99.4% and 91.7% of the traditional process in 40 and 30 min, respectively.

Fig. 2. (a) Schematic illustration ofa new ultra-fast recyclable and value-added desulfation method for spent lead paste via dual RLFRs intensification processes. (b) The flow diagram of the intensification desulfation and lime sulfation process via dual RLFRs intensification processes. (c) Comparison of traditional desulfation and intensification desulfation based on a new RLFR. (d) Particle size distribution of spent lead paste and desulfation lead paste. (e) Particle size of spent lead paste and desulfation lead paste.
The reaction between PbSO4 and (NH4)2CO3 is a solid-liquid phase reaction, with most of the reaction occurring at the interface. Based on the kinetic process analysis (Fig. 3), the desulfation process of spent lead paste is still controlled by internal diffusion. Therefore, the intensification effect of shearing, mechanical grinding, and ultrasonic crushing is the key to improving the desulfation rate of lead paste.
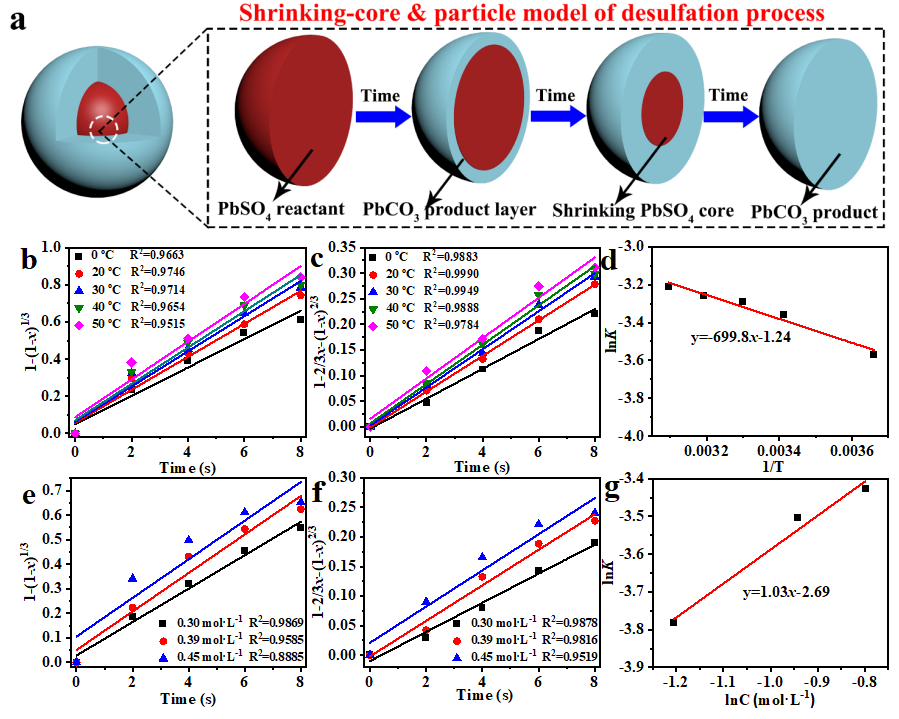
Fig. 3. Dynamic analysis of the desulfation process. (a) Schematics of shrinking-core & particle model for the desulfation mechanism. Curves fitting of (b) chemical reaction and (c) internal diffusion governing equations at different temperatures. (d) The relationship between 1/T and lnK. Curves fitting of (e) chemical reaction and (f) internal diffusion governing equations at different concentrations. (g) The relationship between lnK and lnC.
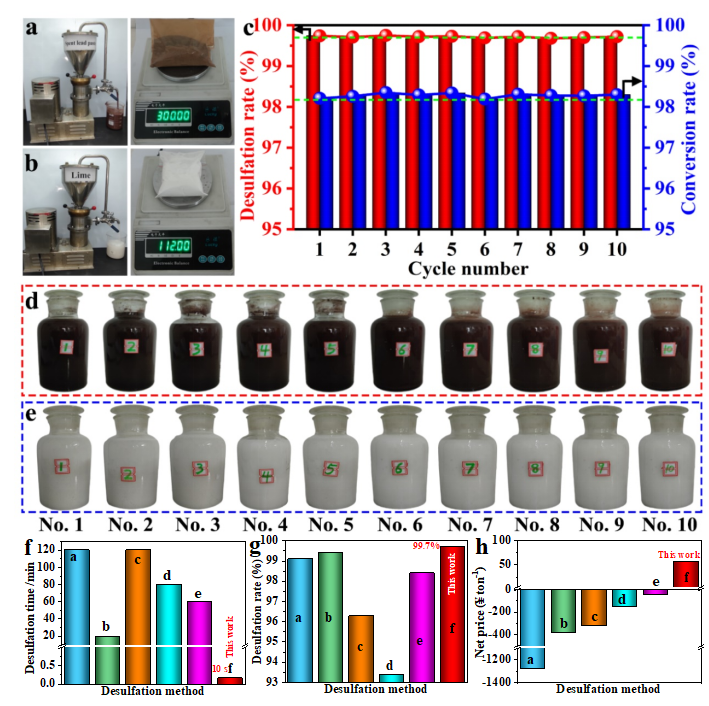
In order to demonstrate the process amplification effect and the recyclability of the mother liquor of RLFR, we have taken 300 g per batch of spent lead paste and 112 g per batch of Ca(OH)2 as raw material and operated 10 batches of continuous intensification desulfation and lime sulfation processes under optimal conditions (Fig. 4). Meanwhile, the average desulfation rates are as high as 99.7% and the average conversion rates of Ca(OH)2 are as high as 98.2% in the 10 batches, which confirms that the intensification desulfation process and the lime intensification sulfation process are stable and feasible, promoting and inspiring the green and low-carbon treatment of other solid waste industries. Furthermore, the new process directly generates high value-added gypsum (CaSO4) by-product, removing the need for evaporation cost of the sulfate solution, which is the positive value-added.
Fig. 4.(a) Photo of the RLFR device for intensification desulfation process of spent lead paste and raw material of spent lead paste (mass: 300 g); (b) Photo of the RLFR device for intensification sulfation process of lime and raw material of lime (mass: 112 g); (c) Desulfation rate and conversion rates of 10 batches by the new dual intensification processes based on the RLFR; Photos of 10 batches of (d) desulfation lead paste and (e) CaSO4 products by the new dual intensification processes based on the RLFR. Comparison of primary economic benefits in (f) desulfation time, (g) desulfation rate, and (h) net price of different desulfation processes, a: Acetic acid-sodium cirtrate desulfation, b: NaOH desulfation, c: Na2CO3 desulfation, d: NH4HCO3 desulfation, e: (NH4)2CO3 desulfation, f: This work.