
Single-atom catalysts (SACs) have been widely applied for diverse catalytic reactions, including CO2 reduction, hydrogenation of olefins and alkynes, water-splitting, catalytic oxidation, and enzymatic reactions. However, the high surface energy of SACs usually requires them to coordinate with C/N/O elements, which benefits their atomic dispersion. Notably, the coordination environment, in particularly the local coordination number (CN), largely affects the electronic structure of SACs. This influences the interactions between SACs and reactants or intermediates, resulting in variations on the efficiency and selectivity of catalytic reactions.
The extended X-ray absorption fine structure (EXAFS) spectroscopy is usually a golden standard for determining the chemical environments around SACs, such as surrounding bond length, CN, and valence state, etc. However, the scarcity of synchrotron X-ray source largely inhibits the wide applications of EXAFS technique. In addition, the later data analysis requires complicated mathematical fitting with specialized training. These drawbacks limit the rapid CN measurement by EXAFS technique. Therefore, it is highly required to develop a simple and rapid method for probing the CN of SACs.
Herein, Prof. Lu and co-workers have developed a d-band center-regulated acetone cataluminescence (CTL) probe for a rapid screening of the CN of Pt-SACs. The universality of proposed acetone-CTL probe was verified by determining the CN ofFe-SACs. This work has opened a new avenue for exploring an alternative to synchrotron XAS for the determination of CN of SACs and even conventional metal catalysts through d-band center-regulated CTL.
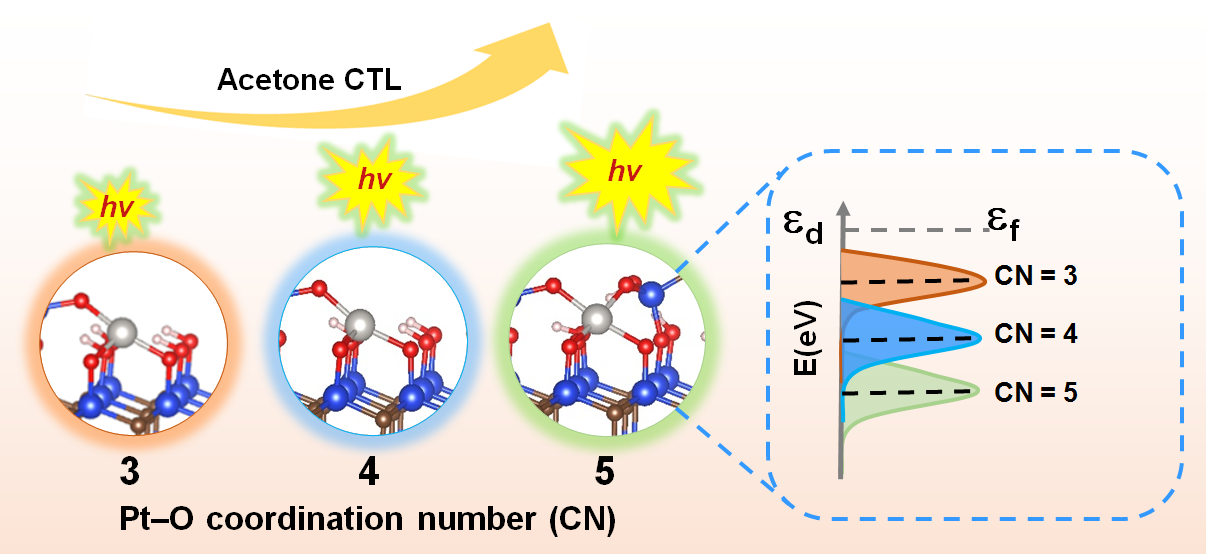
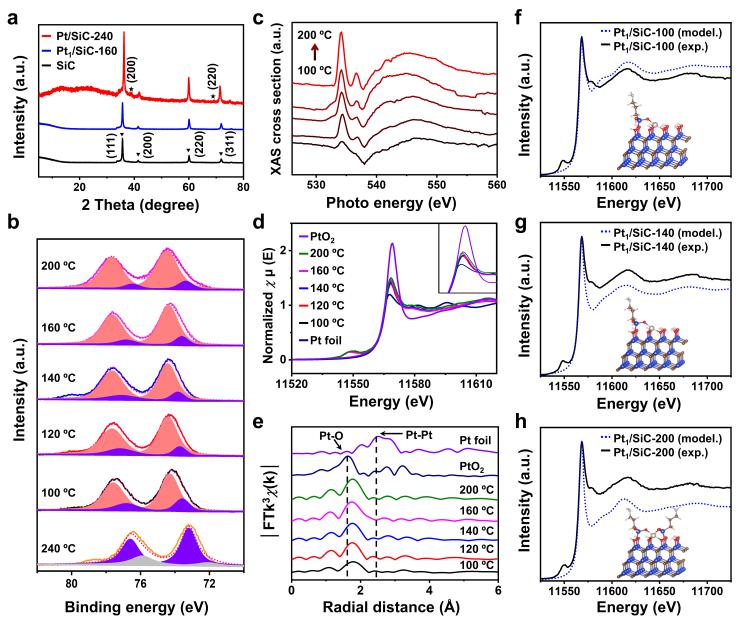
Pt1/SiC SACs with different Pt-O CN have been prepared through a calcination procedure. The detailed dispersity, electronic structure and coordination environment of Pt1/SiC SACs were characterized with XRD, XAS, XPS, EXAFS, and theoretical calculations.
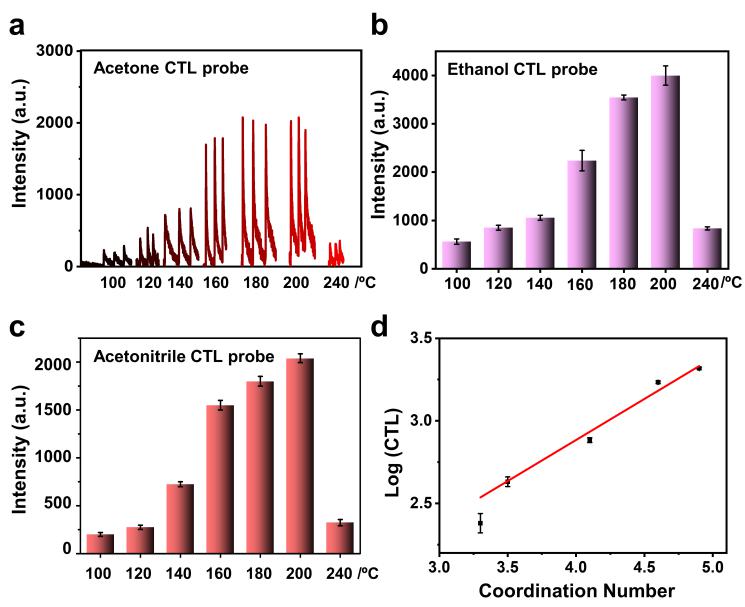
The Pt-SACs-mediated acetone CTL was proportional to the CN. The improved CTL was attributed to the fact that incremental CN of Pt-O could decrease the electron density around Pt atom, accompanying with a downward shift of d-band center in Pt. The downward shift of d-band center facilitates the interaction between the d-orbital electrons and the electron cloud of the reactant. These features benefit the adsorption of reactant and promote the subsequent catalytic reaction.
This work not only exhibits a novel technique for screening the CN of SACs, but also offers a visualized understanding of the relationship between CN and catalytic performances in SACs. Our findings demonstrate the possibility of microstructure or microenvironment characterization toward SACs by d-band center-mediated catalytic process, and might be further applied to tailor the electronic structures of SACs.
Article Information:
Probing Coordination Number of Single Atom Catalysts by d-Band Center-Regulated Luminescence
First Author: Assoc. Prof. Weijiang Guan and Dr. Weiwei Cheng
Corresponding Author: Prof. Zhiqin Yuan and Prof. Chao Lu
Angewandte Chemie International Edition DOI: 10.1002/anie.202401214
