
The dynamic structure evolution of heterogeneous catalysts during reaction has gained great attention recently. However, controllably manipulating dynamic process and then feeding back catalyst design to extend the lifetime remains challenging. Herein, we proposed an entropy variation strategy to develop a dynamic CuZn−Co/HEOs catalyst, in which the non-active Co nano-islands play a crucial role in controlling thermal effect via timely capturing and utilizing reaction heat generated on the adjacent active CuZn alloys, thus solving the deactivation problem of Cu-based catalysts. Specifically, heat sensitive Co nano-islands experienced an entropy increasing process of slowly redispersion during the reaction. Under such heat dissipation effect, the CuZn−Co/HEOs catalyst exhibited 95.7 % ethylene selectivity and amazing long-term stability (>530 h) in the typical exothermic acetylene hydrogenation. Aiming at cultivating it as a catalyst with promising industrial potential, we proposed a simple regeneration approach via an entropy decreasing process.
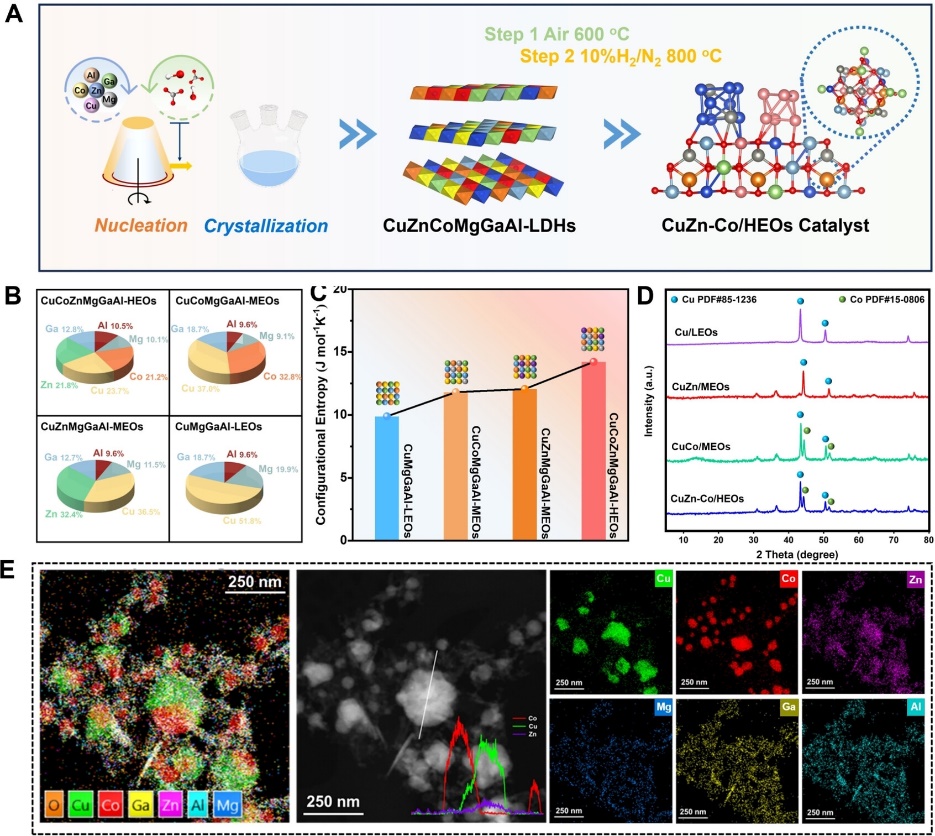
The author prepared CuCoZnMgGaAl high entropy LDHs by nucleation crystallization isolation method, and then calcined them in air to obtain spinel type high entropy oxide (HEOs) precursors. The precursor undergoes entropy reduction under a reducing atmosphere to obtain CuZn-Co/HEOs catalyst. The XRD, XPS, HR-TEM and other characterizations confirmed the formation of heterostructures between CuZn alloy and Co nano metal islands.
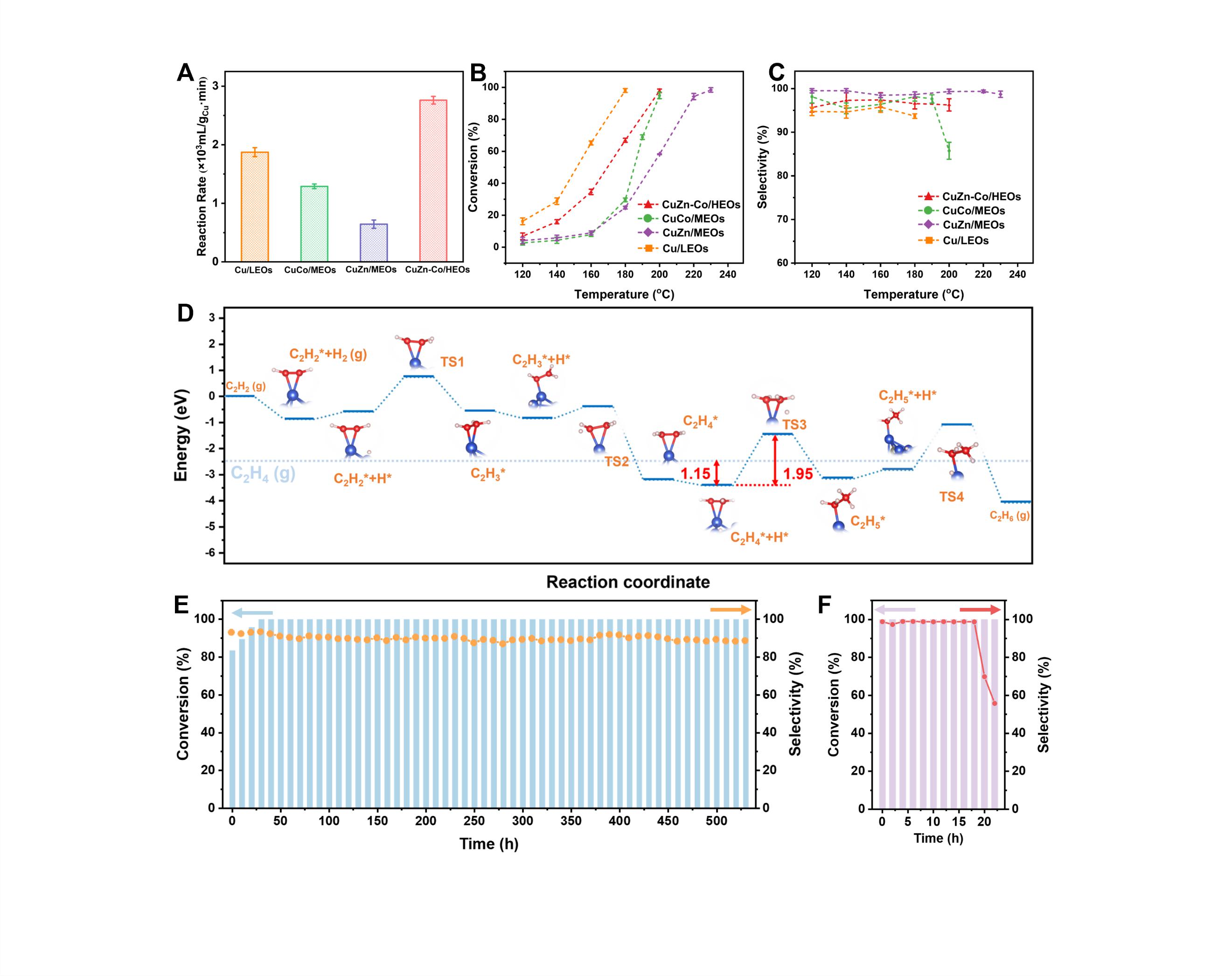
In typical strongly exothermic acetylene selective hydrogenation reactions, CuZn-Co/HEOs catalysts exhibit excellent initial performance and surprising long-term stability. Under the conditions of acetylene conversion rate approaching 100% and ethylene selectivity greater than 95%, the catalyst operated stably for over 530 h. As a comparison, the Cu/LEOs catalyst showed a significant performance decline within 24 h.

The author conducted a series of structural characterizations on CuZn-Co/HEOs that were used for 530 h to explore the reasons for their excellent stability. By comparing the EDS images before and after the reaction, it can be observed that there is a significant redispersion process of Co nano metal islands in the catalyst after use, while the structure of CuZn alloy remains basically unchanged. In the Cu/LEOs catalyst compared with it, a redispersion process of Cu species is observed. Furthermore, the author conducted XAFS energy spectrum characterization of the catalyst before and after the reaction. In the catalyst used for 530 h, the coordination number of Co-Co bonds of Co species decreased significantly while the coordination number of Co-O increased significantly. For Cu species, there was no significant change in the coordination structure before and after the reaction, which is consistent with the results observed in EDS images.

The author further monitored the dynamic evolution process of the catalyst under reaction conditions through in-situ XRD and XPS experiments and explored the driving forces behind the evolution process. From the in-situ XRD spectrum, it can be seen that as the external heat supply temperature increases, the characteristic peak of Co species preferentially undergoes an evolution process of decreasing intensity and gradually increasing half peak width over Cu species. This indicates that compared with Cu species, Co species are more prone to structural evolution of particle size reduction under the action of heat. Furthermore, in the in-situ XPS spectra obtained by extending the holding time at the reaction temperature, it can be observed that the proportion of Co2+ species gradually increases with the extension of reaction time, while the electronic state of Cu species remains basically unchanged, indicating that Co species may have undergone a redispersion process during the reaction. In addition, the author compared the proportion of Co2+ species increase under nitrogen atmosphere and reaction atmosphere, demonstrating that reaction heat is more likely to drive the structural evolution of Co species. At the same time, the author also explored the influence of structural evolution process on the selective hydrogenation catalytic performance of acetylene through in-situ infrared experiments. The characteristic peak of by-product ethane was not observed in the in-situ acetylene hydrogenation of CuZn-Co/HEOs catalyst before and after the reaction, while the production of ethane was clearly observed in the Cu/LEOs catalyst after the reaction.
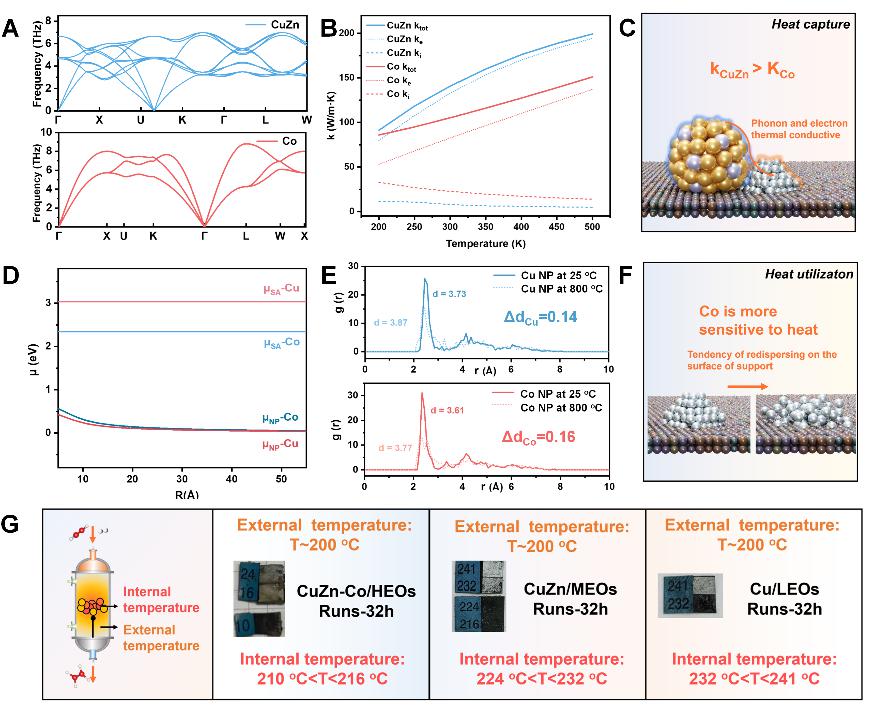
The above experimental results confirm the ability of Co nano metal islands to capture and utilize reaction heat. The author further clarified this process through thermal property calculations and kinetic simulations. Theoretical calculations of the thermal properties of catalysts confirm that the reaction heat generated on CuZn alloys due to differences in thermal conductivity will be captured by nearby Co metal nanoislands in the form of thermally induced phonons and electrons. DFT thermodynamic calculations and AIMD kinetic simulations have confirmed that Co species have stronger thermal sensitivity and are more easily able to utilize heat through the dynamic evolution of their own structure. At the same time, in order to evaluate the thermal management effect of CuZn Co/HEOs catalyst, the authors measured the temperature of the catalytic bed layer using thermosensitive test strips. Experimental data shows that compared with Cu based catalysts without thermal dissipation function, the bed temperature of CuZn Co/HEOs catalyst decreases by about 25 oC.

Based on the above discussion, the author describes a dynamic evolution cycle of catalyst structure constructed based on entropy change strategy. Firstly, Co species capture and utilize the heat of reaction through their own redispersion entropy increase process. In the subsequent regeneration process, the Co species underwent an entropy reduction process of re aggregation, thus completing a cycle of structural dynamic evolution.
Paper Information:Thermal Effect Management via Entropy Variation Strategy to Improve the Catalyst Stability in Acetylene Hydrogenation
The author of the article is doctoral student Zhang Fengyu, and the corresponding author is Professor Feng Junting.
Angew. Chem. Int. Ed. DOI:https://doi.org/10.1002/anie.202412637
