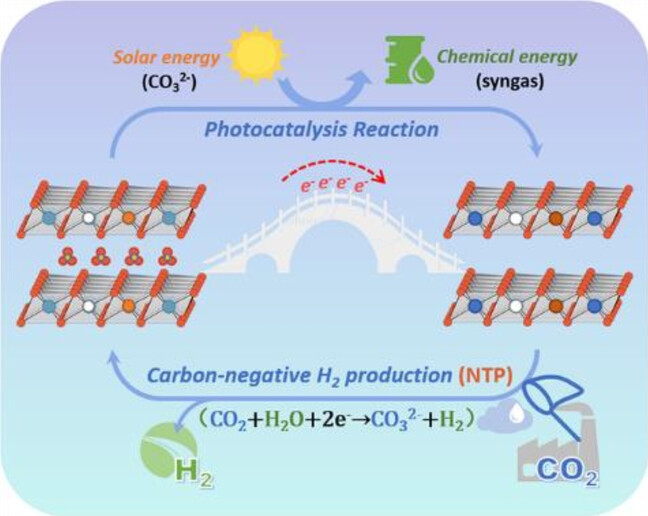
We reported a strategy of carbon-negative H2 production in which CO2 capture was coupled with H2 evolution at ambient temperature and pressure. For this purpose, carbonate-type CuxMgyFez layered double hydroxide (LDH) was preciously constructed, and then a photocatalysis reaction of interlayer CO32− reduction with glycerol oxidation was performed as driving force to induce the electron storage on LDH layers. With the participation of pre-stored electrons, CO2 was captured to recover interlayer CO32− in presence of H2O, accompanied with equivalent H2 production. During photocatalysis reaction, Cu0.6Mg1.4Fe1 exhibited a decent CO evolution amount of 1.63 mmol·g−1 and dihydroxyacetone yield of 3.81 mmol·g−1. In carbon-negative H2 production process, it showed an exciting CO2 capture quantity of 1.61mmol·g−1 and H2 yield of 1.44 mmol·g−1. Besides, this system possessed stable operation capability under simulated flu gas condition with negligible performance loss, exhibiting application prospect.
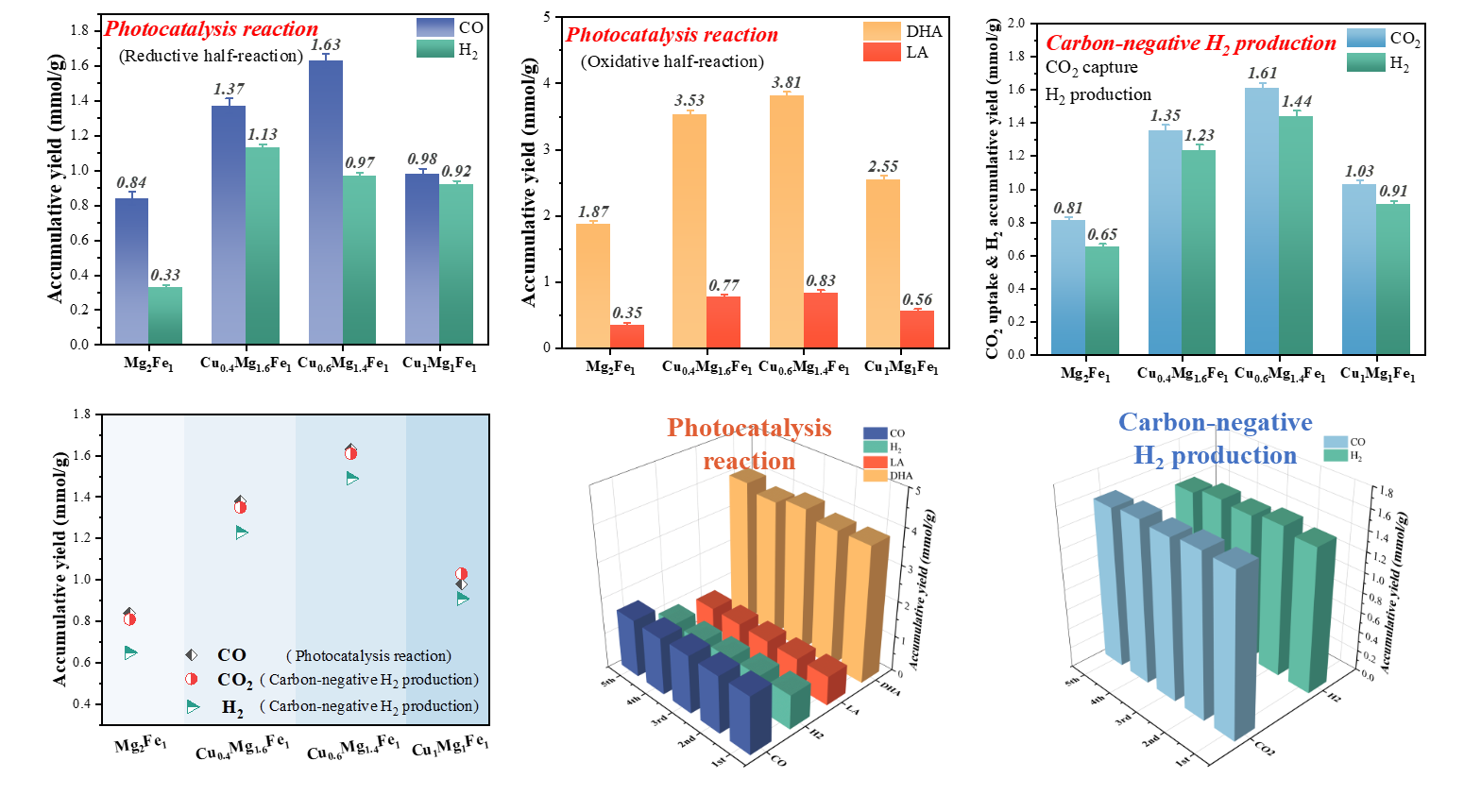
In this study, Professor Feng Junting's team proposed a new process of "negative carbon hydrogen production" driven by photocatalytic reaction based on the principle of process coupling, which combines hydrogen production with CO2 capture and utilization, and develops a new CCU technology under the promotion of sustainable development. Based on the structural characteristics of LDH, a CO32- type CuxMgFex layered double hydroxide (LDH) adsorption catalyst was synthesized. The photocatalytic reaction was driven by interlayer CO32- reduction and glycerol oxidation, and the LDH layer was induced to store electrons. Among them, the CO precipitation amount of Cu0.6Mg1.4Fe1 is 1.63 mmol·g-1, and the yield of dihydroxyacetone is 3.81 mmol·g-1. In the presence of water, pre stored electrons participate in capturing CO2 and restoring the interlayer CO32- form, while producing an equal amount of H2. Under normal temperature and pressure without light, the CO2 capture rate is 1.61 mmol·g-1, and the cumulative H2 yield is 1.44 mmol·g-1.

Further DFT calculations revealed the CO2 reduction reaction pathway and mechanism at the main active sites of Fe photoreduction. At the same time, it was calculated that the average free energy of the CO2 capture and hydrogen production coupling process was -0.41 eV. Therefore, from a thermodynamic perspective, it was verified that the coupling of negative carbon hydrogen production process is spontaneous at ambient temperature and pressure.

Therefore, a new strategy of "negative carbon hydrogen production" has been proposed. At first, the catalyst is in a stationary state (CT0 state) and transitions from the CT0 state to the activated state (CT1 state) through photocatalytic reactions. Among them, glycerol molecules react with photo generated holes to release protons, followed by a reduction reaction of CO32- intercalated molecules (CO32-+4H++2e- → CO+H2O), in which the d orbitals of Cu and Fe cations with redox activity capture additional photo generated electrons, resulting in a corresponding change in valence state and realizing the pre storage process of electrons (Mn++e- → M(n-1)+). To restore the catalyst to its initial resting state, the catalyst can capture CO2 molecules and convert them into interlayer CO32-, H2O re oxidizes the valence changing cations on the layer, resulting in 2M(n-1)++H2O+CO2→ 2Mn++H2+CO32- at ambient temperature and pressure. At this point, the "negative carbon hydrogen production" driven by photocatalytic reaction completes a cyclic process. In addition, the system exhibits high stability under simulated flue gas conditions containing SO2 and NOx, with negligible performance loss, and has broad application prospects.
Paper Information:A Carbon-Negative Hydrogen Production Strategy: CO2 Selective Capture with H2 Production
The author of the article is doctoral student Gao Mingyu, and the corresponding author is Professor Feng Junting.
Angew. Chem. Int. Ed. DOI:https://doi.org/10.1002/anie.202216527
