Professor Song's team published important scientific research progress in Nature Materials
As one of the partners, Prof. Song's team from Beijing University of Chemical Technology, Prof. Pu from Nanyang Technological University ( NTU ) and Prof Zhang's team from Shanxi Provincial People's Hospital published the latest research article entitled “Molecular radio afterglow probes for cancer radiodynamic theranostics” in the international top journal Nature Materials On September 4 (Beijing time).
The result reports the first type of organic luminophores (IDPAs) that can emit near-infrared afterglow and produce 1O2 after X-ray irradiation and apply them for precision cancer theranostics. The in vivo radioafterglow of IDPAs is >25 times brighter than reported inorganic nanophosphor, while the radiodynamic production of 1O2 is >5.5 times higher than the commercially available radiosensitizers. The modular structure of IDPAs allows to develop a smart molecular probe (MRAP) that only turns on its radio-afterglow-dynamic function in the presence of a cancer biomarker. Such a biomarker activation of MRAP enables ultrasensitive detection of diminutive tumor (0.64 mm) with superb contrast (tumor-to-background ratio = 234) and tumor-specific radiotherapy for deep-seated brain tumor with molecular precision at low dosage. Thus, our work not only reveals the molecular guidelines towards organic radioafterglow agents but also highlights new opportunities for cancer radio-theranostics.

![]()

Research background
Optical imaging agents have been pervasively applied in biology and medicine, providing molecular specificity for diagnosis and phototherapy. However, light-tissue interactions limit the imaging and therapeutic abilities of optical agents to superficial or endoscopically accessible tissues. Thereby, afterglow agents that can store photoenergy and release photons after cessation of light irradiation have emerged to mitigate this issue. Due to the elimination of tissue autofluorescence, such photoafterglow agents can detect biomarkers in the deep tissue with high sensitivity. Compared with laser, X-ray has deeper tissue penetration, allowing high-energy photons to be transmitted to the deep lesions of the human body for diagnostic imaging and cancer radiotherapy. Despite the potential of RAI and RDT in cancer theranostics, only inorganic nanophosphors are available, and their radio-afterglow-dynamic function is always-on, limiting their detection specificity and treatment efficacy.
Research results
Synthesis and afterglow mechanism of organic luminophores
The radio-afterglow-dynamic luminophores were synthesized based on the acceptor-phenoxy adamantylidene scaffold via a convergent approach. Then, these acceptors were respectively linked to the phenoxy-adamantylidene with or without the heavy atom (Iodine), leading to the corresponding iodine-containing counterparts, IDPAX (X = N, O, Su and Se) (Figure 1a). All the compounds were verified by nuclear magnetic resonance, and liquid chromatography-mass spectrometry. These data reveal that the radioafterglow mechanism of DPAs and IDPAs is closely associated with radiodynamic process (Figure 1b). First, X-ray irradiation of DPA or IDPA leads to the radiodynamic generation of 1O2. Then, the in-situ generated 1O2 reacts DPA or IDPA via cycloaddition reaction and converts it into a dioxetane intermediate. At last, this intermediate gradually decomposes to the corresponding activated products (activated DPA or IDPA) meanwhile releasing photons. Although both luminophores generated 1O2 under X-ray irradiation, the presence of heavy iodine in IDPAs can not only enhance their X-ray absorption but also efficiently populate triplet excitons. Thus, IDPAs radiodynamically produced more 1O2 than the iodine-free counterparts, which facilitated the formation of dioxetane intermediate and consequently emitted stronger radioafterglow.
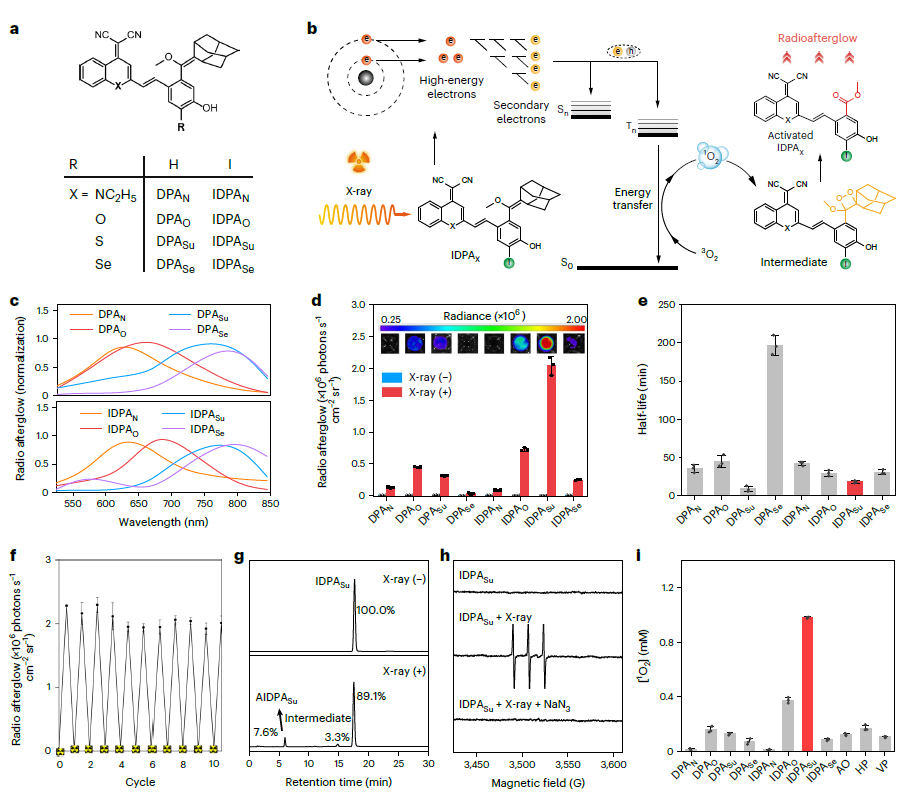
Figure 1. Synthesis and characterization of radio-afterglow-dynamic luminophores.
Imaging and treating cancer deeper
The radio-afterglow-dynamic processes of IDPAs are inducible at a record tissue-depth of 15 cm. Due to its smart biomarker-triggered radioafterglow, the probe sets a record-high tumor-to-background ratio of 234 that is even 5.7 times higher than an inorganic nanophosphor emitting second NIR light. Such a biomarker activation of the probe not only enables ultrasensitive detection of diminutive tumor (0.64 mm) with superb contrast, but also brings the specificity of radiotherapy to molecular level.
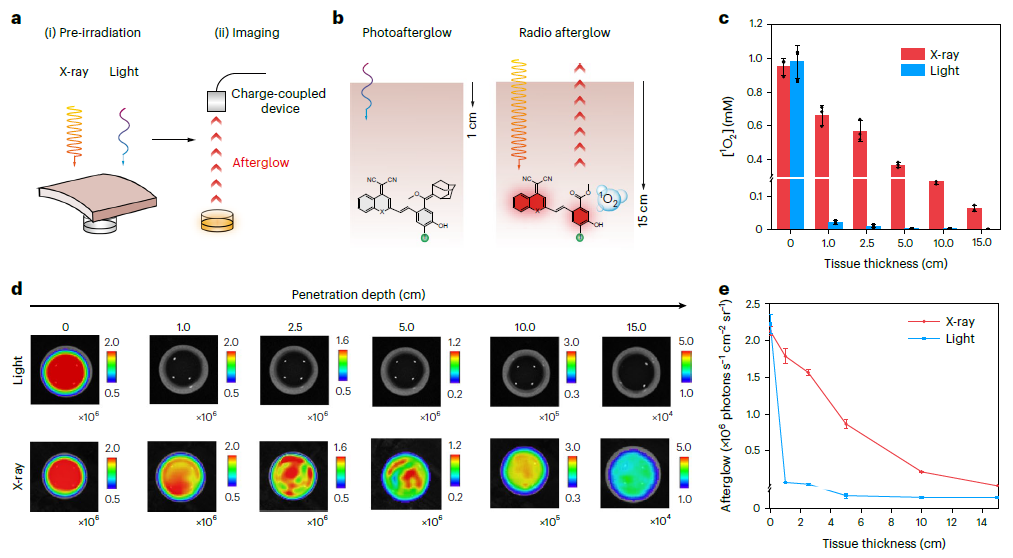
Figure 2. Deep-tissue radio-afterglow-dynamic process.
Precision therapy with minimal dosage
The probe can inhibit the deep brain tumor growth at a radiation dosage of 3.2 Gy, which is at least 6.7 lower than reported radiodynamic agents (>20 Gy).
To enable precision cancer diagnosis and therapy, IDPASu was modified into a molecular radio-afterglow dynamic probe (MRAP) that only activated its radioafterglow and radiodynamic function in the presence of a tumor biomarker, Cathepsin B (CatB). The biomarker-activatable radiodynamic effect of MRAP was validated by ESR, showing the ESR signal of 1O2 increase by 7.8-fold upon incubation of MRAP with CatB followedby X-ray irradiation. Thereby, the survival time for MRAP-mediated RDT groups were increased from 37 (PBS) to 76 days, showing a 100% of survival rate until 67 days.

Figure 4. MRAP-mediated radioafterglow imaging and radiodynamic therapy.
Conclusion and Prospect
In summary, we have developed a first type of organic radio-luminophores that outperform inorganic nanophosphors to emit brighter NIR radioafterglow and autonomously produce radiodyanmic 1O2 without additives. The modular structure of these radio-luminophores facilitated precise control of their physiological properties and construction of a smart molecular probe (MRAP) with unprecedented biomarker-activated radio-afterglow-dynamic function. Such a biomarker specificity of MRAP allowed to detect diminutive tumor with superb contrast and perform radiotherapy for deep-seated tumor with molecular precision at low dosage. Thus, our work not only reveals molecular guidelines to fill in the gap of organic radioafterglow agents but also highlights a new translational direction towards precision theranostics.
Personal Profile:
Jibin Song obtained his PhD degree in Chemical and Biomedical Engineering from Nanyang Technological University, Singapore, in 2014. Then he worked with Prof. Xiaoyuan (Shawn) Chen as a postdoctoral fellow at the National Institutes of Health (NIH). He joined the Beijing University of Chemical Technology as a Professor of analytical chemistry. Prof. Song has published over 150 papers in high impact journals. His research mainly focuses on developing molecular imaging nanoprobes for bioimaging, biosensing and drug/gene delivery.
