
Electrocatalytic semihydrogenation of alkynols to alkenols under ambient conditions using H2O as a hydrogen source is highly attractive in synthetic chemistry. However, it is still challenging to achieve high Faradaic efficiency (FE) in a wide potential window. Herein, we reported a bimetallic Cu3Au alloy as an efficient catalyst for electrocatalytic semihydrogenation of alkynols to alkenols. Specifically, during semihydrogenation of 2‐butyne‐1,4‐diol (BYD) to 2‐butene‐1,4‐diol (BED), the Cu3Au catalyst achieves 12.6-fold greater reaction rate and higher FE compared with pure Cu (99% vs. 63%). Moreover, the Cu3Au maintains >96% FEs in a wide potential window from −0.19 to −0.59 V vs. RHE. We demonstrate that the competitive adsorptions of reactive hydrogen (H*) and BYD greatly influence the semihydrogenation processes. The presence of Au in Cu3Au facilitates H* formation and reduces BYD adsorption on Cu, thus enhancing BYD hydrogenation performance. The Cu3Au catalyst affords a broad substrate scope from alkynols to aromatic alkynes, producing corresponding alkenes in good selectivities. Finally, we coupled BYD semihydrogenation with glycerol oxidation to replace oxygen evolution reaction in a two-electrode system, showing 40% energy saving at 200 mA for BED production and co-production of valuable formate at anode, demonstrating an economical manner.
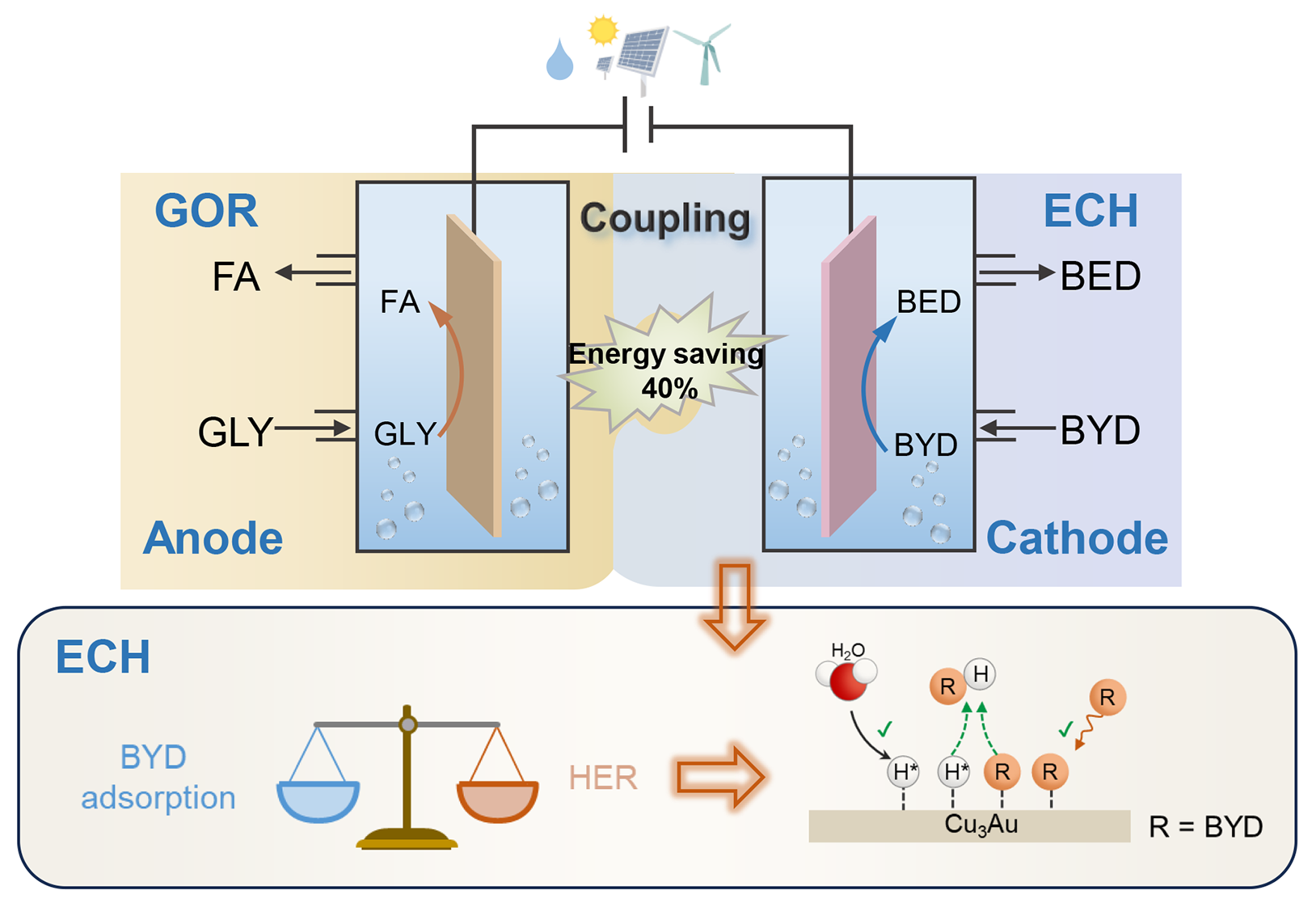
Schematic of the main components of this article.
ACS Catalysis DOI: 10.1021/acscatal.3c05928
