Professor Shao Mingfei’s team at our college has published an article in Angew. Chem. Int. Ed.:Evaluation of Active Oxygen Species Derived from Water Splitting for Electrocatalytic Organic Oxidation.

The active oxygen species (OH*/O*) generated through the electrolysis of water are crucial for the electrocatalytic oxidation of organic compounds into high-value chemicals. The formation of these active oxygen species on the catalyst surface is a key determinant of the activity and selectivity of electrooxidation of organic compounds. However, the relationship between the formation of active oxygen species and the activity and selectivity of electrocatalytic oxidation reactions remains ambiguous. In this work, we have chosen the electrocatalytic oxidation of glycerol as a model reaction to systematically study the relationship between the activity and selectivity of glycerol electrooxidation and the formation energy of the active oxygen species OH* (ΔGOH*).
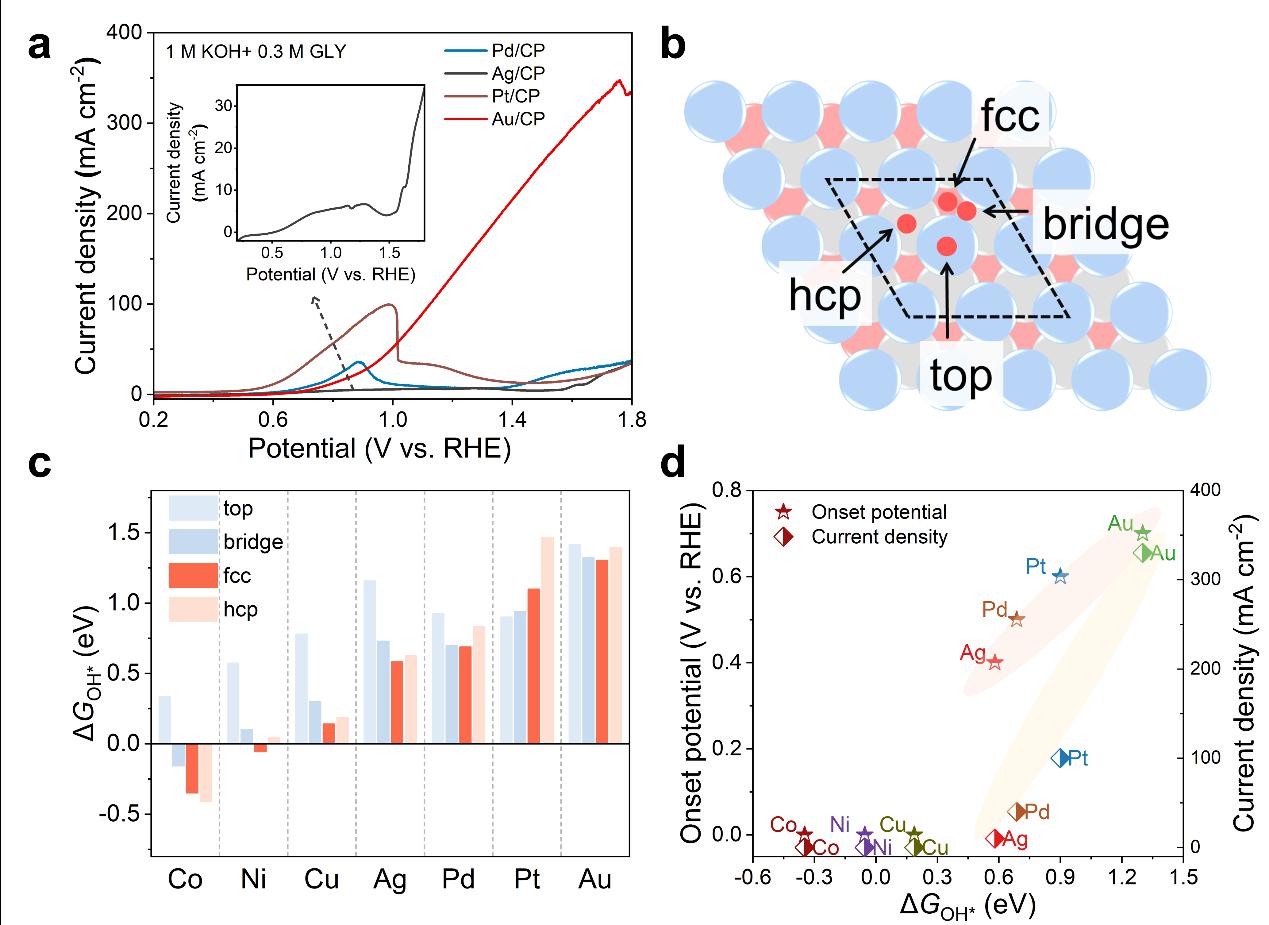
Figure 1.a) LSV curves of Pd, Ag, Pt, and Au electrodes at a scan rate of 10 mV/s in 1 M KOH with 0.3 M GLY; more detailed LSV curves of Ag in the inset. b) Schematic of four adsorption sites (top, bridge, face-centered cubic (fcc), and hexagonal close-pack (hcp)) for the formation of OH* on the metal (111) surfaces (metal = Co, Ni, Cu, Pd, Ag, Pt, and Au). c) The ΔGOH* at four sites on the metal (111) surfaces (metal = Co, Ni, Cu, Pd, Ag, Pt, and Au). d) Near line relationship between onset potential and current density of GLY electrooxidation reaction with the optimal ΔGOH* over metals (metal = Co, Ni, Cu, Pd, Ag, Pt, and Au).
The fcc or hcp sites on metal surfaces are more favorable for the formation of OH*, and there is a near-linear relationship between the optimal ΔGOH* on the (111) surfaces of seven metals (Co, Ni, Cu, Ag, Pd, Pt, and Au) and the onset potential and current density for the electrooxidation of glycerol. Au exhibits better performance in glycerol oxidation and has stronger resistance to overoxidation compared to the other metals.
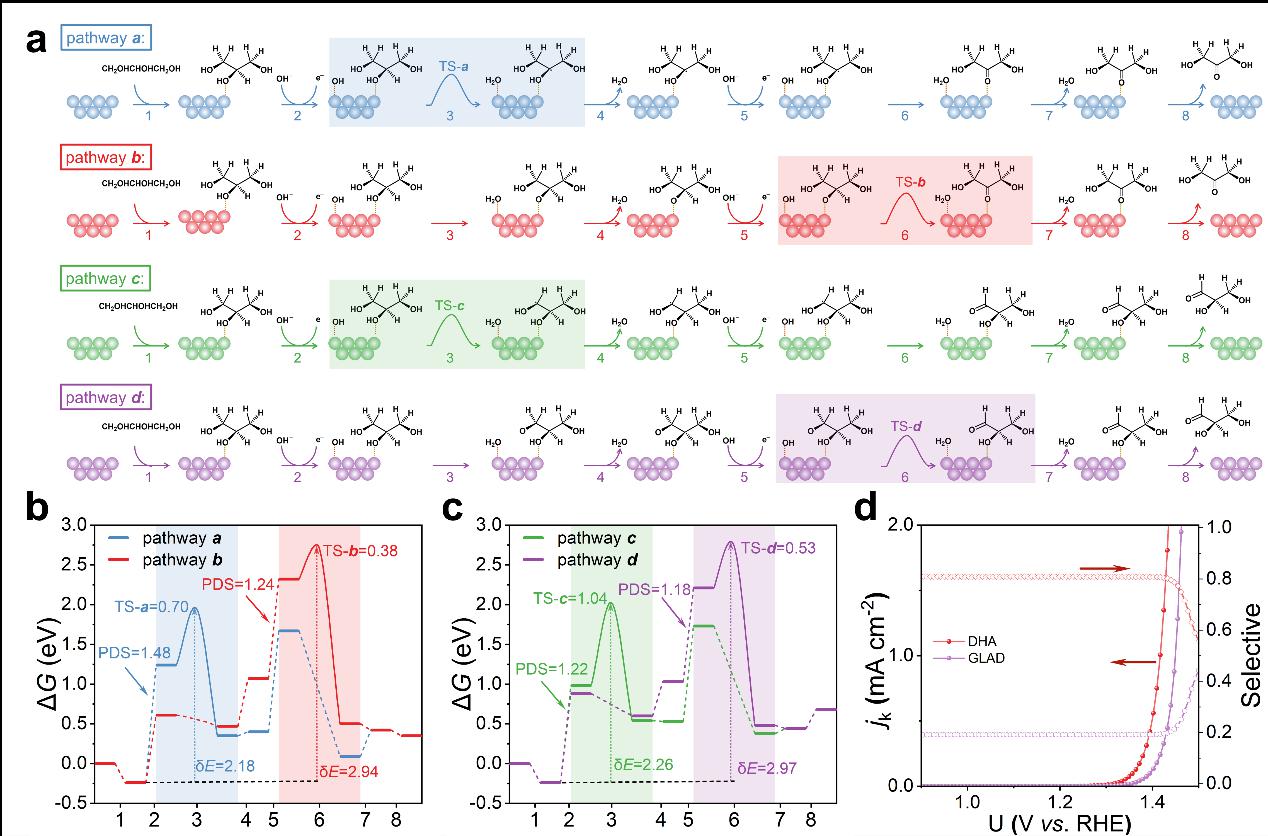
Figure 2.a) Schematic illustration of the elementary reaction process for the GLY electrooxidation to DHA (pathways a and b) and GLAD (pathways c and d) on the Au(111) surface. b) Gibbs free energy diagrams for the GLY electrooxidation to DHA (pathways a and b) on the Au(111) surface at 0 V vs. RHE. c) Gibbs free energy diagrams for the GLY electrooxidation to GLAD (pathways c and d) on the Au(111) surface at 0 V vs. RHE. The PDS, energetic span (δE) and transition states are labeled in the diagrams. The corresponding detailed reaction equations and results are listed in Tables S3 and S4. d) Kinetic current density (jk) and selectivity of GLY electrooxidation to DHA and GLAD through microkinetic analysis.
The generation process of the active oxygen species (OH*) is the potential determining step of the entire reaction, and the activation energy for breaking the C−H bonds of different intermediates varies. The oxidation of glycerol to DHA is kinetically more favorable.
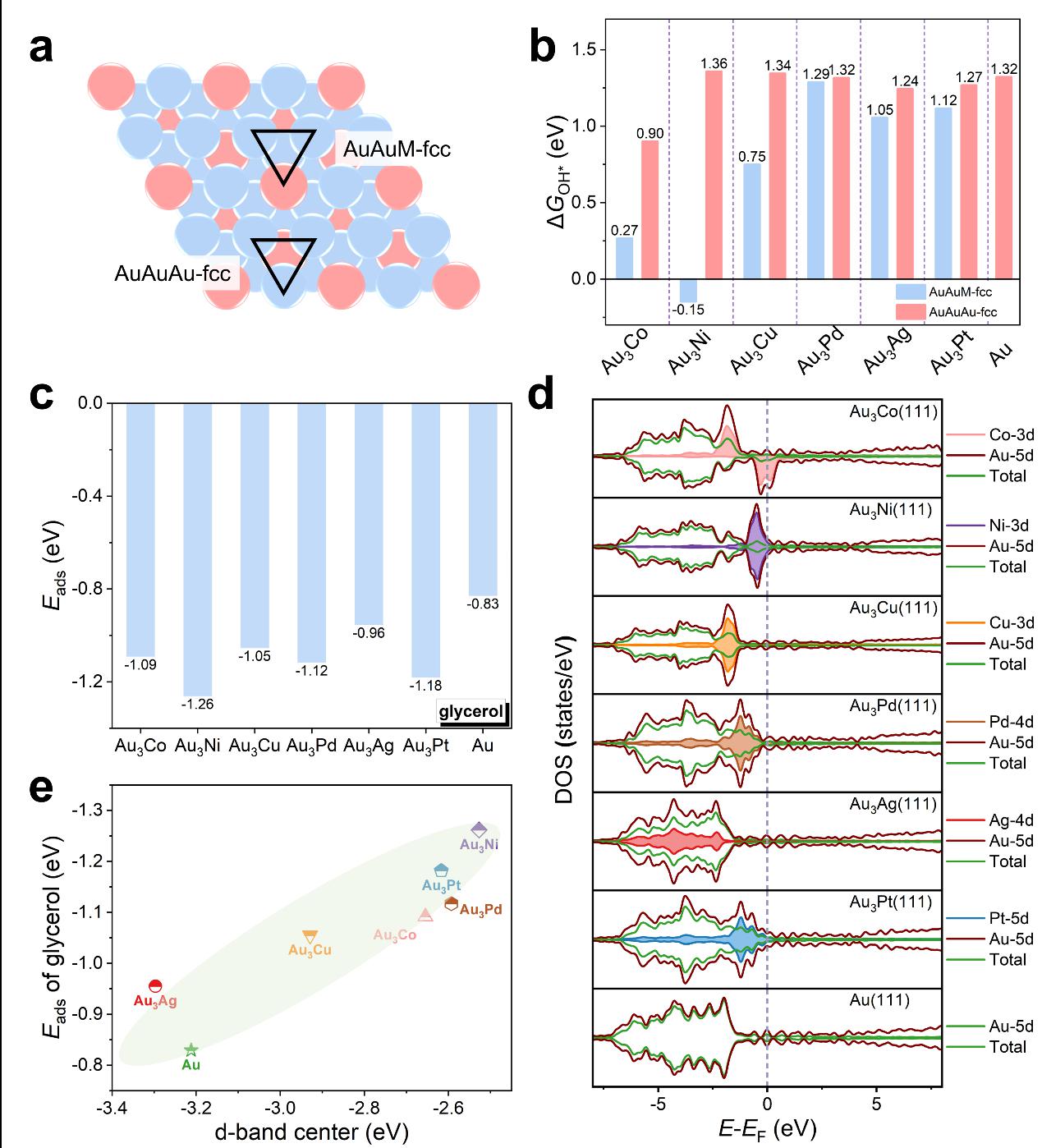
Figure 3.a) Schematic of two different coordination structures for OH* formation on the Au3M(111) surfaces: the AuAuM-fcc site involved an doped metal atom and two Au atoms, and the AuAuM-fcc site involved three Au atoms. b) The ΔGOH* at two fcc sites on the Au3M(111) and Au(111) surfaces. c) The Eads of GLY on the Au3M(111) and Au(111) surfaces. d) The DOS of the 3d orbitals of Co, Ni, and Cu, the 4d orbitals of Pd and Ag, and the 5d orbitals of Pt and Au in Au3M alloys, respectively. e) The relationship between the d-band center of the Au3M(111) and Au(111) surfaces and the Eads of GLY on the Au3M(111) and Au(111) surfaces.
Metal doping reduces the formation energy of active oxygen species OH* on the one hand and enhances the adsorption energy of the reactant glycerol on the other hand.
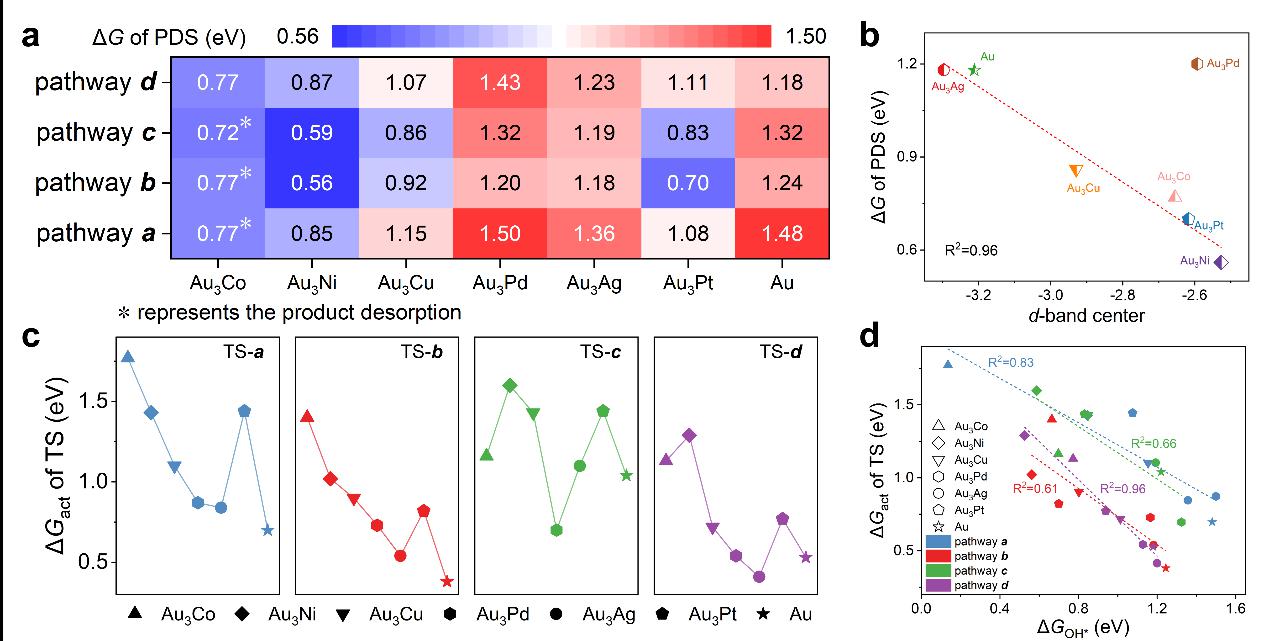
Figure 4.a) The ΔG of the PDS for the reaction pathways a, b, c and d of GLY electrooxidation on the Au3M(111) and Au(111) surfaces. b) The linear relationship between the ΔG of the optimal PDS of the reaction pathway among pathways a, b, c and d of GLY electrooxidation on the Au3M(111) and Au(111) surfaces. c) The ΔGact of the transition states for the C−H bond cleavage reaction catalyzed by OH* in reaction pathways a, b, c and d on the Au3M(111) and Au(111) surfaces. d) The linear relationship between the ΔGact of the transition states for the C−H bond cleavage reaction and the corresponding ΔGOH* in reaction pathways a, b, c and d on the Au3M(111) and Au(111) surfaces.
Thermodynamic analysis indicates that the potential-determining step for the electrooxidation of glycerol on the alloy surfaces is the generation of the active oxygen species (OH*); kinetic analysis reveals an inversely proportional relationship between the activation barrier of the C-H bond cleavage and the corresponding formation energy of the active oxygen species (OH*).
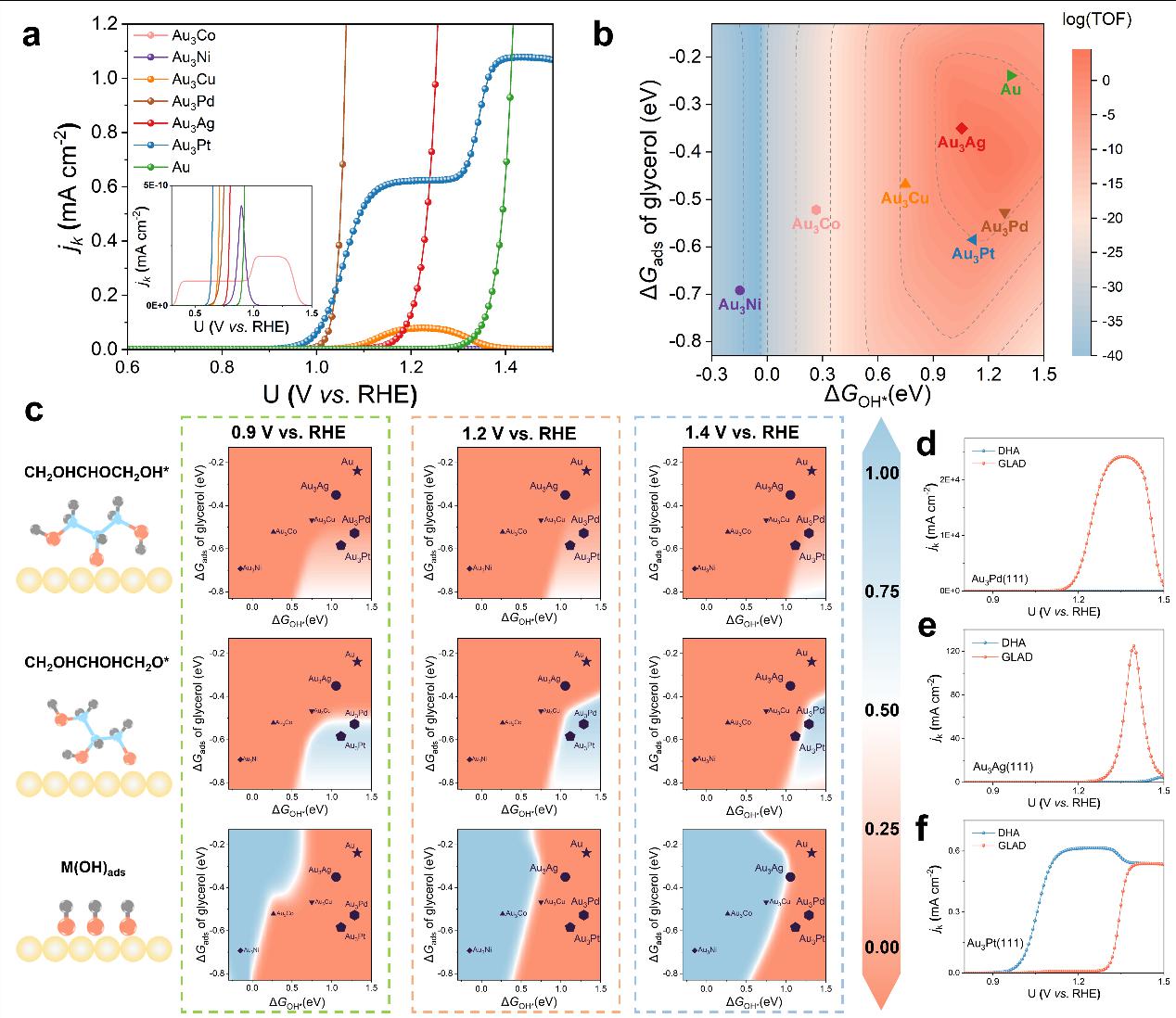
Figure 5.a) Kinetic current density (jk in mA cm−2) of the GLY electrooxidation over the Au3M(111) and Au(111) surfaces in microkinetic analysis. b) Theoretical reaction rate (TOF in s-1) of GLY electrooxidation at 1.4 V vs. RHE. Values of TOF on a logarithmic scale. c) The coverage of intermediates CH2OHCHOCH2OH*, CH2OHCHOHCH2O* and OH* on Au3M(111) and Au(111) surfaces at 0.9, 1.2 and 1.4 V vs. RHE. d) Kinetic current density (jk in mA cm−2) of DHA and GLAD on the Au3Pd(111) surface. e) Kinetic current density (jk in mA cm−2) of DHA and GLAD on the Au3Ag (111) surface. f) Kinetic current density (jk in mA cm−2) of DHA and GLAD on the Au3Pt(111) surface.
Taking into account both the thermodynamics and kinetics of the glycerol electrooxidation reaction, a detailed microkinetic model was established, revealing a volcano relationship between the activity of the glycerol electrooxidation reaction and the formation free energy of OH* (ΔGOH*) as well as the adsorption free energy of glycerol (ΔGads). Au3Pd and Au3Ag, which are close to the volcano peak, possess moderate ΔGOH* and ΔGads and exhibit superior activity.
Paper Information: Evaluation of Active Oxygen Species Derived from Water Splitting for Electrocatalytic Organic Oxidation
The co-authors of this article are Yang Jiangrong and Xia Tian, doctoral students from the Class of 2022, with Professor Shao Mingfei and Professor Li Zhenhua being the corresponding authors.
Angewandte Chemie International Edition. DOI: 10.1002/anie.202413457
