Organic solar cells are rapidly becoming the next generation of clean energy technologies due to their light weight, mechanical flexibility, low cost, and the ability to be processed in large areas with solution-based methods, which hold broad application prospects. At present, phosphonic acid-based self-assembled materials show advantages comparable to traditional hole extraction materials like PEDOT:PSS. However, the structure-performance relationship between their molecular structures and device performance is still unclear. Therefore, clarifying the relationship between their self-assembly behavior and optoelectronic performance from a molecular structural perspective is of great significance for constructing efficient and stable organic photovoltaic cells.
Based on this, Professor Liu Yao's research group at the Soft Matter High-End Innovation Center designed and synthesized four new types of "non-fused ring dipodal phosphonic acids" molecular materials (TBT-L, TBT-L-Br, TBT-A, and TBT-A-Br, collectively referred to as NFR-DPAs), characterized by their "thiophene-benzene-thiophene" type skeleton and dipodal phosphonic acid anchoring groups. Among them, TBT-L and TBT-A are configurational isomers with linear and angular skeletons, corresponding to C2h and C2v symmetry, respectively. After bromination, they transform into TBT-L-Br and TBT-A-Br, with molecular structures and corresponding optoelectronic properties as shown in Figure 1.

Figure 1. Molecular structures and optoelectronic properties of NFR-DPAs
This study first used first-principles calculations to simulate the self-assembly behavior of non-fused ring dipodal phosphonic acid molecules on ITO substrates, indicating that the four non-fused ring dipodal phosphonic acids interact with the ITO substrate through strong multidentate binding at two anchoring points, showing configuration-dependent molecular orientation. Compared with angular skeleton (C2v symmetry) and non-brominated non-fused ring dipodal phosphonic acid molecules, those with a linear structure (C2h symmetry) and brominated skeleton exhibit good planar orientation and stronger ability to modify the electrode work function. This orientation is beneficial for enhancing interfacial charge transfer transport, thereby helping to reduce charge recombination and promote hole extraction. Polarization-modulated infrared reflection absorption spectroscopy (IRRAS) and near-edge X-ray absorption fine structure spectroscopy (NEXAFS) characterization provided direct experimental evidence of the interfacial self-assembly orientation of non-fused ring dipodal phosphonic acid molecules, revealing the structure-performance relationship between configurational isomerization and molecular interfacial self-assembly behavior. In addition, non-fused ring dipodal phosphonic acid molecules have adjustable anode interfacial modification capabilities, reducing interfacial barriers, improving the vertical phase separation of the active layer, and suppressing interfacial non-radiative recombination.
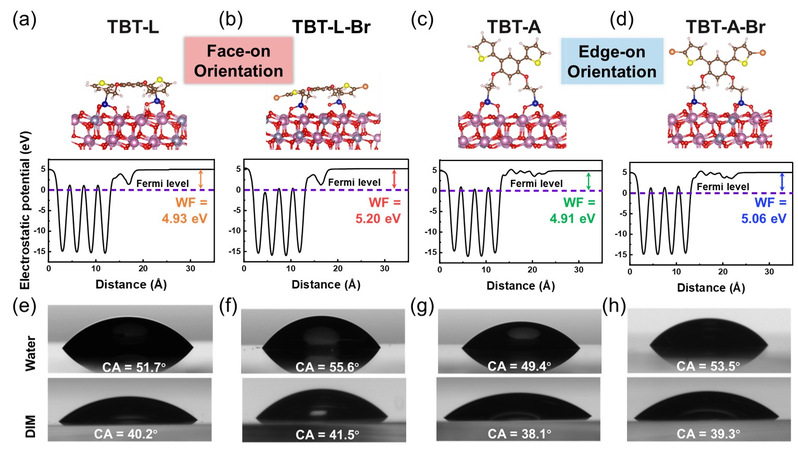
Figure 2. Optimal configurations of NFR-DPAs on ITO calculated by DFT and surface energy characterization
Ultimately, photovoltaic cells modified with brominated and linear symmetric non-fused ring dipodal phosphonic acid molecules achieved a photoelectric conversion efficiency of 19.11% in the binary PM6: L8-BO system and a maximum photoelectric conversion efficiency of 19.66% in the ternary D18:PM6:L8-BO system, outperforming the currently commercialized hole transport material PEDOT:PSS and 2PACz, and exhibiting excellent battery device stability. Moreover, electrodes modified with non-fused ring dipodal phosphonic acid molecules also demonstrate universal compatibility with various organic active layers. This study not only provides a simple method for achieving high-performance organic photovoltaic cells but also offers a molecular-level understanding of the self-assembly behavior of phosphonic acid molecular materials on transparent electrode substrates, which is significant for optimizing the surface morphology and electronic structure of transparent conductive electrodes to achieve efficient organic electronic devices.
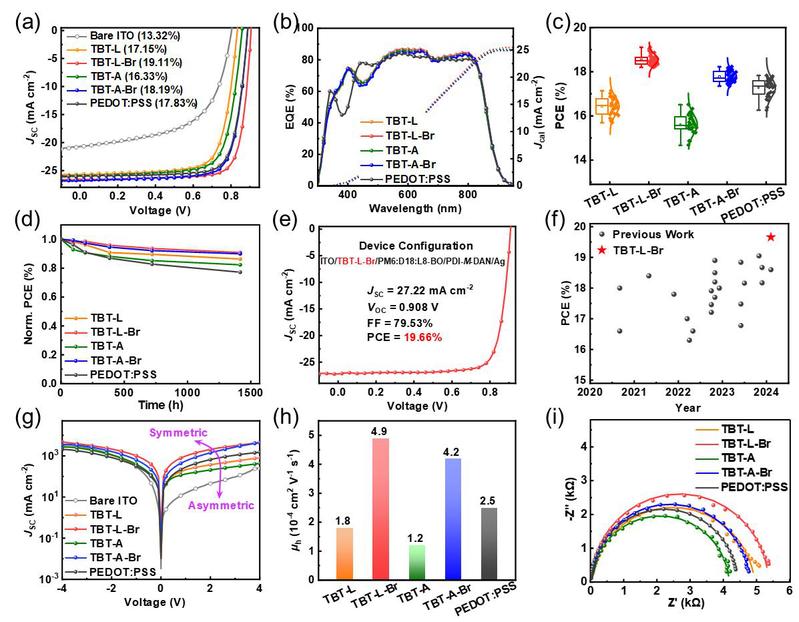
Figure 3. Characterization of the optoelectronic performance of organic photovoltaic devices
