
This work reveals how nanoscale grain boundaries (GBs) enhance the intrinsic surface activity of CeO₂ nanomaterials through electronic mechanisms. Grain boundaries were introduced by thermally decomposing cerium carbonate and cerium formate precursors. X-ray absorption near-edge structure (XANES) spectra at the O K-edge and Ce L₃-edge indicate that the grain boundaries reduce the covalency of Ce–O bonds. H₂ temperature-programmed reduction (H₂-TPR) and Raman spectroscopy further show that this reduction in orbital overlap weakens the lattice potential's constraint on surface oxygen atoms. This electronic effect fundamentally increases the lability of surface lattice oxygen, thereby promoting the formation of oxygen vacancies and the activation of O₂. As a result, benzyl alcohol is selectively oxidized to benzaldehyde with 100% selectivity. This structure–activity relationship, based on decreased lattice covalency, provides a new electronic perspective for understanding how grain boundaries and size reduction enhance the surface reactivity of nanomaterials.
Background
Oxide-based nanomaterials, such as cerium dioxide (CeO₂), play a pivotal role as active components or supports in various catalytic applications, including heterogeneous catalysis, photocatalysis, electrocatalysis, and environmental catalysis. Their involvement in metal–support interactions (MSI), as well as their impact on catalytic activity and selectivity, fundamentally depends on the reactivity of surface lattice oxygen atoms (Oₛₗ). Generally, weaker bonding between Oₛₗ and the oxide lattice favors interfacial bonding and adhesion at the metal–oxide interface, facilitates the release of Oₛₗ to form oxygen vacancies (V_O), promotes the formation of H₂O and CO₂ during catalytic cycles, and enhances O₂ activation to generate reactive oxygen species (ROS). Therefore, tuning the reactivity of Oₛₗ is crucial for optimizing the catalytic performance of oxide nanomaterials.
To this end, various strategies have been developed to regulate their surface reactivity, including controlling particle size, morphology, and exposed crystal facets; doping with main-group metals (e.g., Ga), transition metals (e.g., Cu), or rare-earth elements (e.g., La); and introducing defects such as V_O, strain, or grain boundaries (GBs). Despite advances in synthesis and modification, a deep understanding of the physical and chemical mechanisms—particularly at the electronic level—underlying the intrinsic reactivity of oxide nanomaterials remains lacking. This represents a fundamental scientific question critical for understanding the catalytic and redox properties of oxide-based catalysts.
Highlights of this study
The researchers elucidated the role of reduced Ce–O covalency—a concept that describes the degree of orbital overlap and electron sharing in covalent bonds—in governing the intrinsic reactivity of surface lattice oxygen (Oₛₗ) on CeO₂ nanomaterials. This was achieved by introducing nanoscale grain boundaries (GBs), a type of two-dimensional (2D) defect, into the CeO₂ lattice to weaken the Ce–O covalent bonding. While GBs have been reported to enhance the catalytic activity of metal and metal oxide catalysts, the specific mechanism by which such 2D defects modulate surface reactivity to promote O₂ activation has remained unclear. This study demonstrates that nanoscale GBs can effectively reduce the covalency of Ce–O bonds by disrupting the periodicity of the CeO₂ lattice.
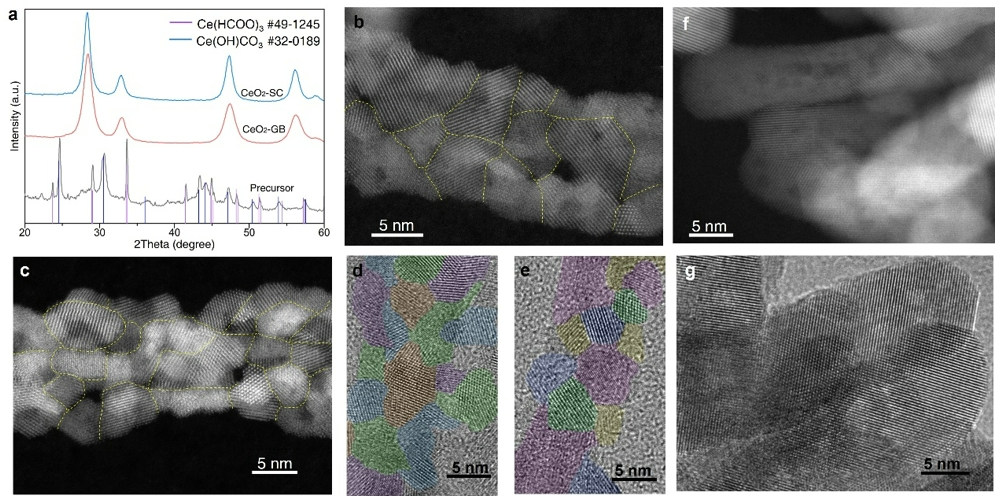
Figure 1. Structural characterization of CeO2 nanorods rich in nanoscale GBs.
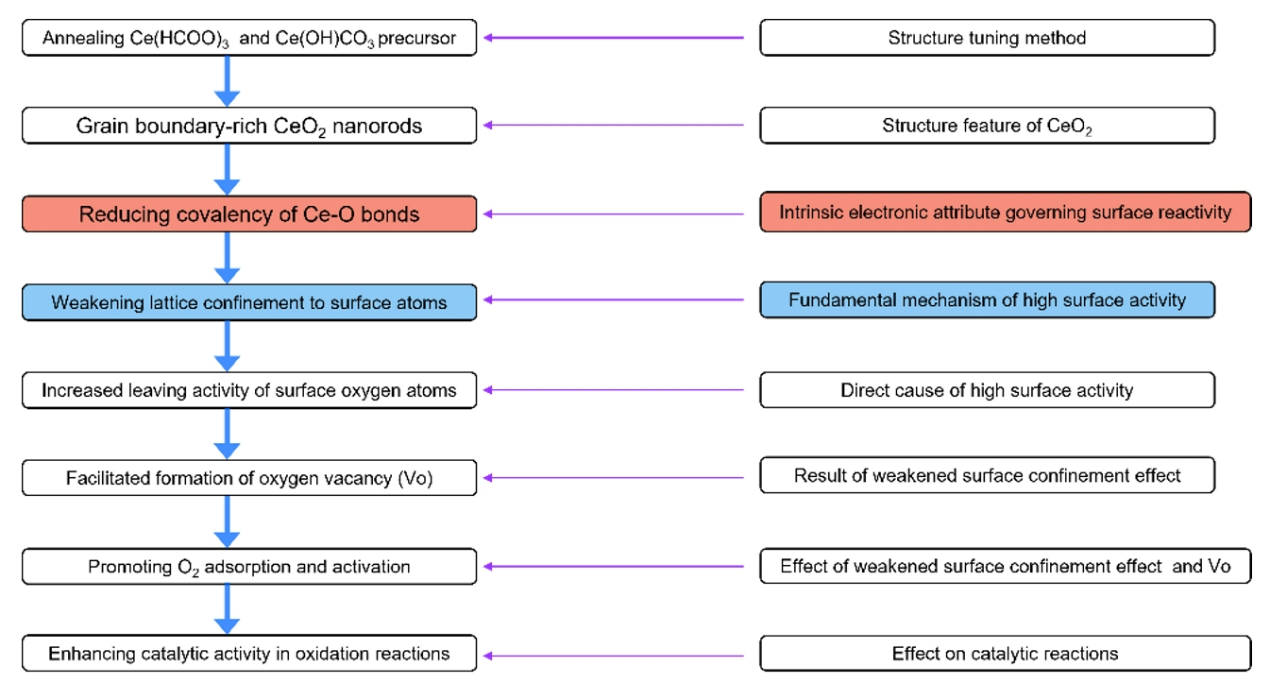
Cerium oxide nanorods enriched with grain boundaries (CeO₂-GB) were successfully synthesized via thermal decomposition of a precursor composed of cerium formate and cerium hydroxycarbonate. This precursor was obtained by heating cerium nitrate (Ce(NO₃)₃) in ethylene glycol (EG) at 180°C. Transmission electron microscopy (TEM) images revealed that the precursors were nanorods with an average diameter of approximately 20nm. High-resolution TEM (HRTEM) images exhibited clear lattice fringes, while the X-ray diffraction (XRD) pattern (Figure 1a) showed sharp peaks, indicating high crystallinity. The diffraction peaks could be indexed to a mixture of Ce(HCOO)₃ (JCPDS 49-1245) and Ce(OH)CO₃ (JCPDS 32-0189). Experimental results highlighted the critical role of nitrate ions (NO₃⁻) in controlling the phase composition and morphology of the precursors. By varying the amount of Ce(NO₃)₃ from 0.5g to 10.0g, it was found that at lower concentrations (<1.0g), CeO₂ nanoparticles were obtained. At higher concentrations (>4.0g), NO₃⁻ acted as an oxidizing agent, converting EG into formate and carbonate species, which facilitated the formation of Ce(HCOO)₃ and Ce(OH)CO₃ nanorods.
The XRD pattern (Figure 1a) confirmed the formation of pure CeO₂, with broad peaks suggesting small grain sizes. Scanning transmission electron microscopy (STEM) images (Figures 1b–1c) of the calcined CeO₂-GB nanorods revealed a porous architecture composed of polycrystalline nanoparticles. HRTEM images (Figures 1d–1e) further showed grain sizes in the range of 3–6nm and distinct lattice misorientations, confirming the presence of grain boundaries. For comparison, single-crystal CeO₂ nanorods without grain boundaries (CeO₂-SC, Figures 1a and 1f–1g) were synthesized via a hydrothermal reaction in a sodium hydroxide solution. In addition, commercial CeO₂ nanoparticles (CeO₂-NP) with an average diameter of 22.0±2.0nm were used as another reference. The CeO₂-SC nanorods exhibited extended lattice continuity without disruption by grain boundaries, as confirmed by HRTEM (Figures 1f–1g).
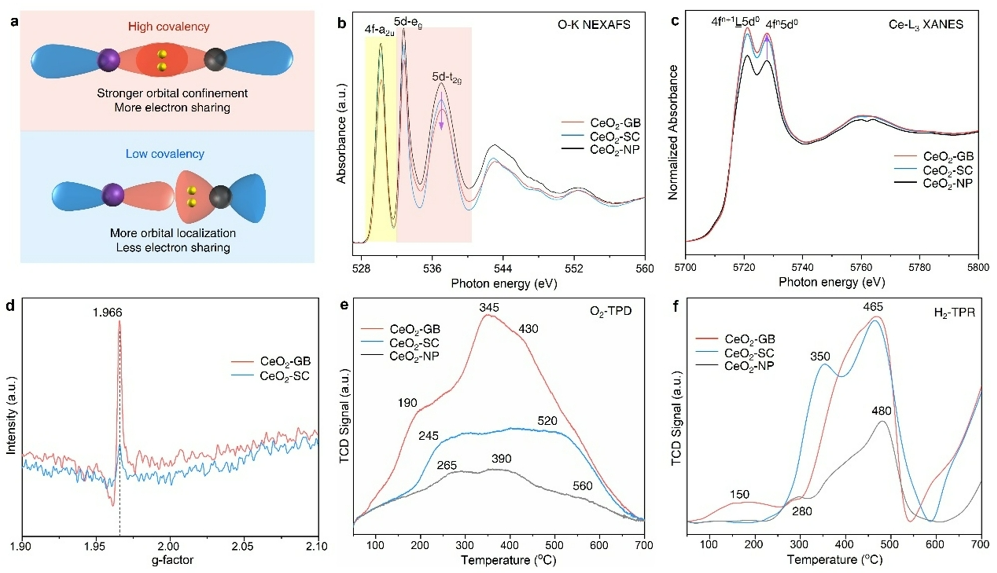
Figure 2. Probing reduced Ce−O covalency with XAFS and reactive SOS.
The researchers employed X-ray absorption near-edge structure (XANES) spectroscopy to investigate the effect of grain boundaries (GBs) on the electronic structure of CeO₂ nanomaterials. XANES is capable of probing the density of unoccupied valence states, which reflects both the degree of covalency between orbitals and electrons, as well as the binding strength of these orbitals and electrons (Figure 2a). In nanomaterials with high covalency, the covalent bonds exhibit lower polarization, resulting in a greater degree of electron sharing and delocalization between atoms. Conversely, in materials with reduced covalency, more polarized or even ionic bonds are formed, wherein electrons become more localized around the more electronegative atom.
In metal oxides such as CeO₂, lower covalency leads to decreased electronic sharing between O 2p orbitals and the metal valence orbitals (e.g., Ce 5d). This interaction trend reduces the electron occupancy of Ce 5d orbitals while increasing that of O 2p orbitals, resulting in a weakened XANES signal at the O K-edge and a strengthened signal at the Ce L-edge. Higher covalency favors electron delocalization, whereas reduced covalency enhances electron localization on non-metal atoms. This shift in bonding character consequently alters the overall electronic structure of the nanomaterial.
Figure 3. Probing GB-boosted catalytic activity with benzyl alcohol (BA)oxidation.
The researchers employed the oxidation of benzyl alcohol (BA) as a model reaction to investigate the influence of covalency reduction, induced by grain boundaries (GBs), on the catalytic activity of CeO₂. Pure CeO₂-GB and CeO₂-SC were used as catalysts, and the reactions were conducted under both N₂ and O₂ atmospheres without solvent dilution of the BA substrate. The results showed that CeO₂-GB consistently exhibited higher turnover frequencies (TOFs) across all tested temperatures, with TOFs increasing as temperature rose, indicating that the presence of GBs enhanced the oxidative capability of CeO₂.
Moreover, under an O₂ atmosphere, the TOFs of CeO₂-GB were significantly higher than those under N₂, suggesting that CeO₂-GB could more effectively activate O₂ at lower temperatures and thus promote the oxidation reaction. In addition, the apparent activation energies (Ea) of CeO₂-GB under both N₂ and O₂ conditions were lower than those of CeO₂-SC, further confirming that the introduction of GBs enhanced the generation of surface reactive oxygen species (ROS) and the ability to activate O₂. In summary, GBs effectively boost the intrinsic surface reactivity of CeO₂ nanomaterials and improve their catalytic performance.
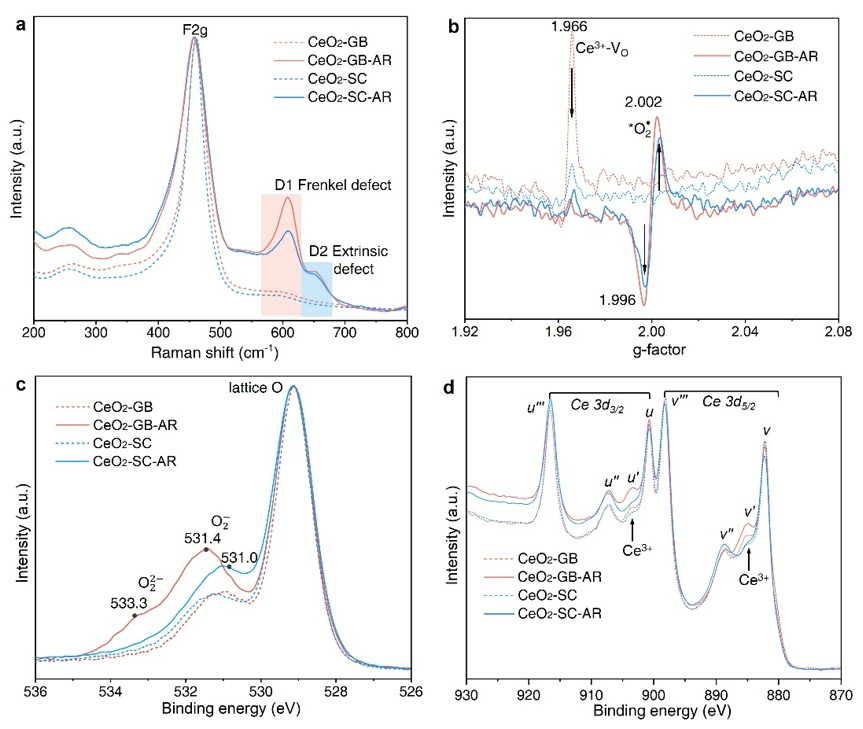
Figure 4. Defect states of CeO2 materials after BA oxidation.
Following the oxidation of benzyl alcohol, changes in the defect states of Ce ions were observed. To further compare the defect structures and surface properties of CeO₂-GB and CeO₂-SC, the researchers conducted a series of characterizations—Raman spectroscopy, electron paramagnetic resonance (EPR), and X-ray photoelectron spectroscopy (XPS)—on the materials before and after catalytic testing. Raman spectroscopy is particularly effective in probing defect states in CeO₂ nanomaterials, while EPR is employed to detect surface defect states and reactive oxygen species (ROS) on CeO₂ surfaces. In addition, O 1s and Ce 3d XPS spectra provided complementary evidence.
These characterizations collectively confirmed that CeO₂-GB exhibits higher surface reactivity, characterized by the enhanced formation of oxygen vacancies and Ce³⁺ species.
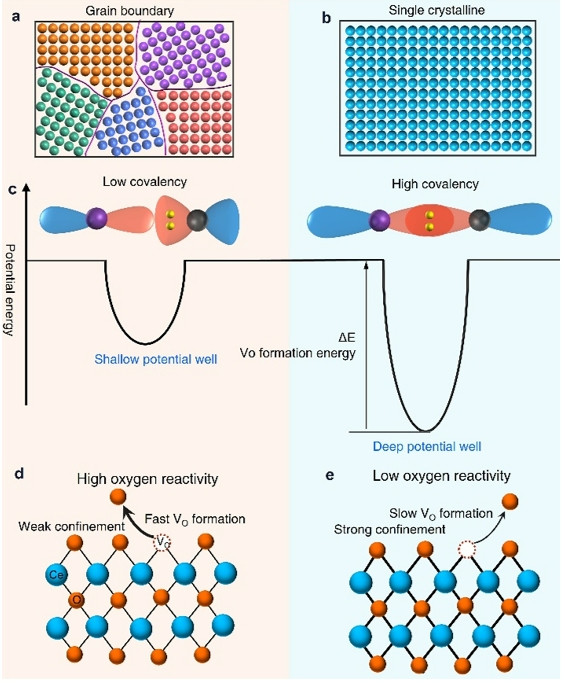
Figure 5. Scheme showing the electronic mechanism of the GB on ceria surface reactivity.
In CeO₂, the presence of grain boundaries (GBs) weakens the Ce–O covalent bonding strength and reduces the binding energy of surface oxygen atoms, thereby facilitating their release and promoting the formation of oxygen vacancies (Vₒ) (Figure 5d). These vacancies serve as active sites for the adsorption and activation of O₂, leading to the generation of reactive oxygen species (ROS) that drive the catalytic oxidation process. By accelerating the formation of Vₒ and enhancing ROS generation, GBs effectively improve the catalytic efficiency of CeO₂, particularly in oxidation reactions.
Moreover, the increased reactivity of surface lattice oxygen fundamentally improves O₂ adsorption and activation, contributing to an overall enhancement in catalytic performance. In contrast, strong Ce–O covalent bonding results in higher binding potentials that suppress the release of surface oxygen atoms (Figure 5e). Therefore, the overall tendency of surface lattice oxygen to dissociate determines the surface reactivity of oxide nanomaterials. GBs intrinsically weaken the binding of surface oxygen atoms by disrupting orbital coupling between adjacent atoms and diminishing the lattice potential wells that stabilize surface species.
Conclusion and Outlook
The researchers employed CeO₂ as a model material to investigate the role of grain boundaries (GBs) in enhancing the surface reactivity of nanomaterials and elucidated the underlying electronic mechanisms. The results demonstrated that the introduction of GBs significantly improves the catalytic activity of CeO₂ and lowers the activation energy in oxidation reactions, such as the oxidation of benzyl alcohol, with a more pronounced effect compared to single-crystalline CeO₂ nanorods.
X-ray absorption near-edge structure (XANES) analysis revealed that nanoscale GBs effectively reduce the covalency of Ce–O bonds by disrupting the long-range orbital coupling associated with the periodicity of the crystal lattice. This reduction in covalency diminishes the potential wells stabilizing lattice and surface atoms, thereby lowering the energy barrier for the release of surface lattice oxygen atoms. The weakened binding facilitates the formation of oxygen vacancies and promotes O₂ adsorption and activation, which in turn enhances the generation of reactive oxygen species (ROS) that are essential for catalytic oxidation processes.
This study highlights that engineering GBs represents an effective strategy for tuning the electronic structure of oxide nanomaterials, providing a promising approach to the design of high-performance catalysts with improved catalytic functionalities.
Reference link:https://pubs.acs.org/doi/10.1021/jacs.5c03536
Author Biography
Zhao Weixin
Zhao Weixin is a Master’s degree holder (graduated in 2021) from the College of Chemistry, Beijing University of Chemical Technology.
Jia Wenyu
Jia Wenyu is currently a postgraduate student (enrolled in 2022) at the College of Chemistry, Beijing University of Chemical Technology.
Xiang Guolei
Xiang Guolei is an Associate Professor at the College of Chemistry, Beijing University of Chemical Technology. He earned his Ph.D. under the supervision of Prof. Wang Xun at Tsinghua University (2008–2014), specializing in inorganic nanomaterial synthesis, and subsequently conducted postdoctoral research at the University of Cambridge (2014–2017). Since 2017, his work has centered on deciphering the electronic structure mechanisms of nanomaterial surface/interface interactions through innovative experimental and theoretical approaches. He pioneered novel concepts such as surface valence orbital competitive reconstruction, orbital potential theory, nanoscale cooperative chemical adsorption, and π-state longitudinal polarization, providing fundamental insights into nanoscale physicochemical processes. His recent publications (past five years) include high-impact papers in Nature Communications, Journal of the American Chemical Society, Nano Letters, and other leading journals.
Wang Xun
Wang Xun is a Professor at Tsinghua University and a leading figure in nanomaterials research. After obtaining his B.S./M.S. from Northwest University (1994–2001) and Ph.D. from Tsinghua University (2004), he joined Tsinghua’s faculty, becoming a full professor in 2007. A recipient of the National Science Fund for Distinguished Young Scholars and the Yangtze River Scholar Distinguished Professorship, he has garnered numerous accolades, including the 2023 Second Prize of the National Natural Science Award (1st contributor), the inaugural Xplorer Prize (2019), and the Royal Society of Chemistry Fellowship (2015). He serves in key editorial roles for Science China Materials, Nano Research, and Advanced Materials, among others. With over 200 SCI publications and >20,000 citations, his research spans nanomaterial synthesis and applications, significantly advancing the field.
