Recently, Professor Shao Mingfei's team at Beijing University of Chemical Technology (BUCT) has made an important breakthrough in the field of electrocatalytic plastic recycling and resource utilization, realizing for the first time the full-flow electrocatalytic conversion of polyethylene terephthalate (PET) wastes to the biodegradable polymer polyglycolic acid (PGA), and achieving 1,000 hours of stable operation at ampere current densities with simultaneous co-production of hydrogen gas.
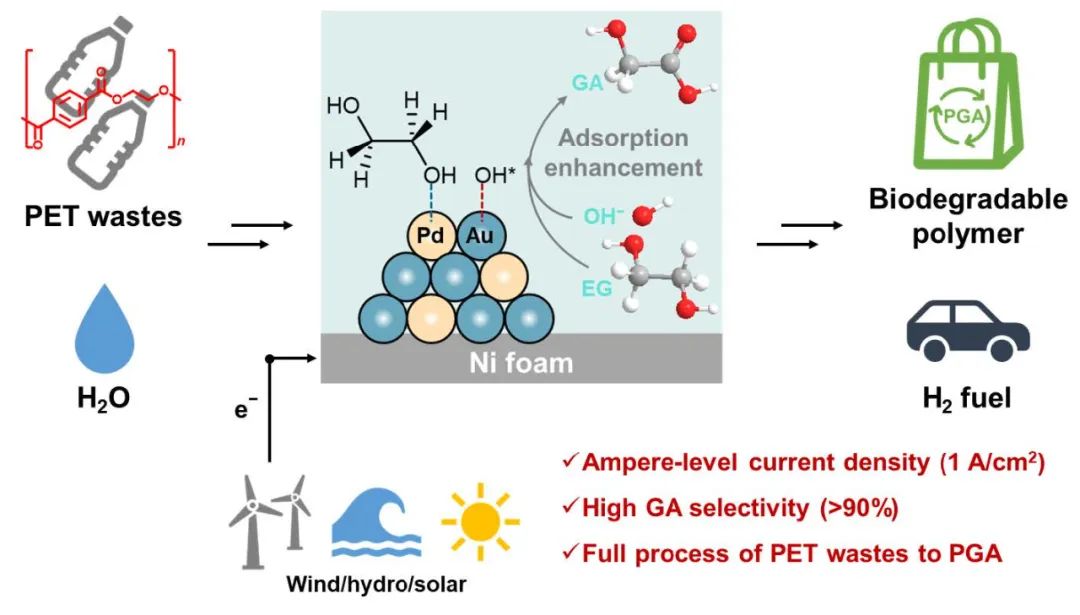
Fig. 1. Conversion of PET waste at high current density to biodegradable PGA and H2
Background:
Plastic pollution has become a global environmental problem, and the annual production of PET, the most widely used polyester plastic, is up to 70 million tons, however, the recycling rate is less than 20%, and a large amount of waste ends up in landfills or oceans, posing a serious threat to the ecosystem. In recent years, electrocatalysis has gradually become a powerful tool for the resource utilization of plastic waste due to its advantages of greenness, mildness and high efficiency. The electrocatalytic conversion of ethylene glycol (EG), a product of PET hydrolysis, to glycolic acid (GA), a biodegradable polymer monomer, has been studied, but there are common problems, such as the difficulty of current density to reach the amperage level and the poor stability of the catalyst, which make it difficult to meet the requirements of practical applications. In addition, the GA product often exists in the form of glycolate, which needs to be neutralized by adding additional acid before it can be used for further polymerization to PGA, which not only increases the complexity of the process, but also significantly increases the cost. Therefore, it is still a major challenge to realize the efficient, stable, and low-energy-consumption full-process conversion of PET waste to biodegradable PGA.
Highlights of this paper:
Prof. Mingfei Shao's team at Beijing University of Chemical Technology (BUCC) has long been committed to the research and application of coupled oxidation systems for hydrogen production from electrolytic water. To address the above challenges, the team first constructed an AuPd alloy electrocatalyst with synergistic active sites, in which Au acts as a catalytic site to promote the generation of reactive oxygen species (OH*), and Pd acts as an adsorption site to enhance the interfacial adsorption and enrichment of EG. Under this synergistic effect, high efficiency EG electrooxidation at amperometric current densities (>1 A/cm2) was achieved, with a GA selectivity as high as 94%, and stable operation for up to 1000 hours, which is significantly better than the reported catalytic system.
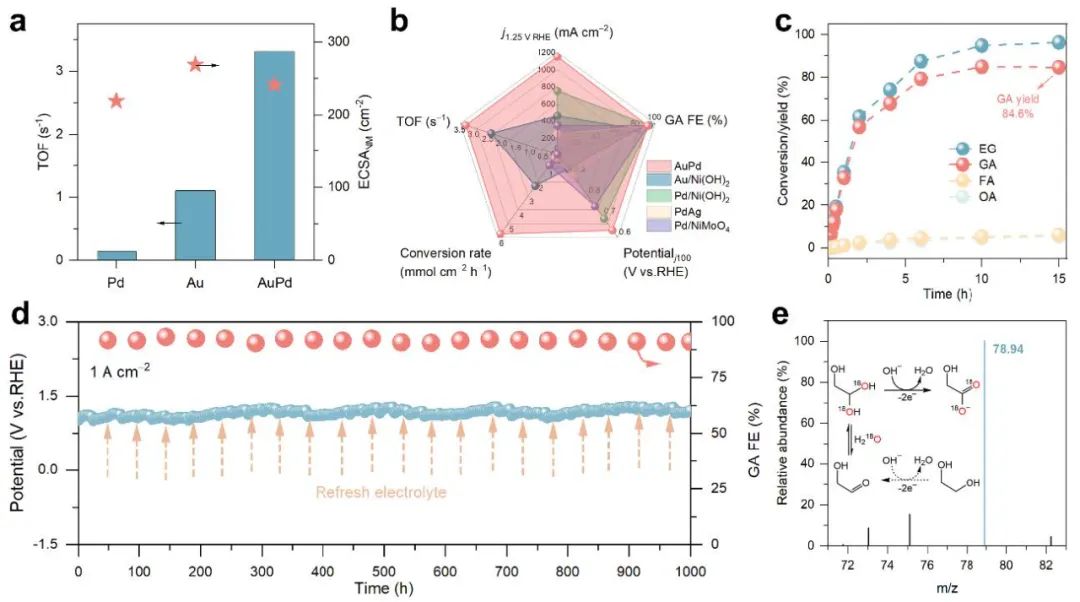
Figure 2. Performance test
The team further assembled a homemade diaphragm-less flow electrolyzer in the lab and achieved an ultra-high EG oxidation current of 35.1 A under simulated industrial conditions and a tank pressure of 1.4 V, with a GA yield of 515.4 g/day and a hydrogen yield of 14.3 L/h. Moreover, electrodialysis was introduced to efficiently separate the ethanoic acid product, and to realize the recovery of excess KOH in the electrolyte, with a high recovery rate of 97%. Combined with the rotary evaporation process, high purity GA crystals (selectivity up to 95%) were finally obtained.
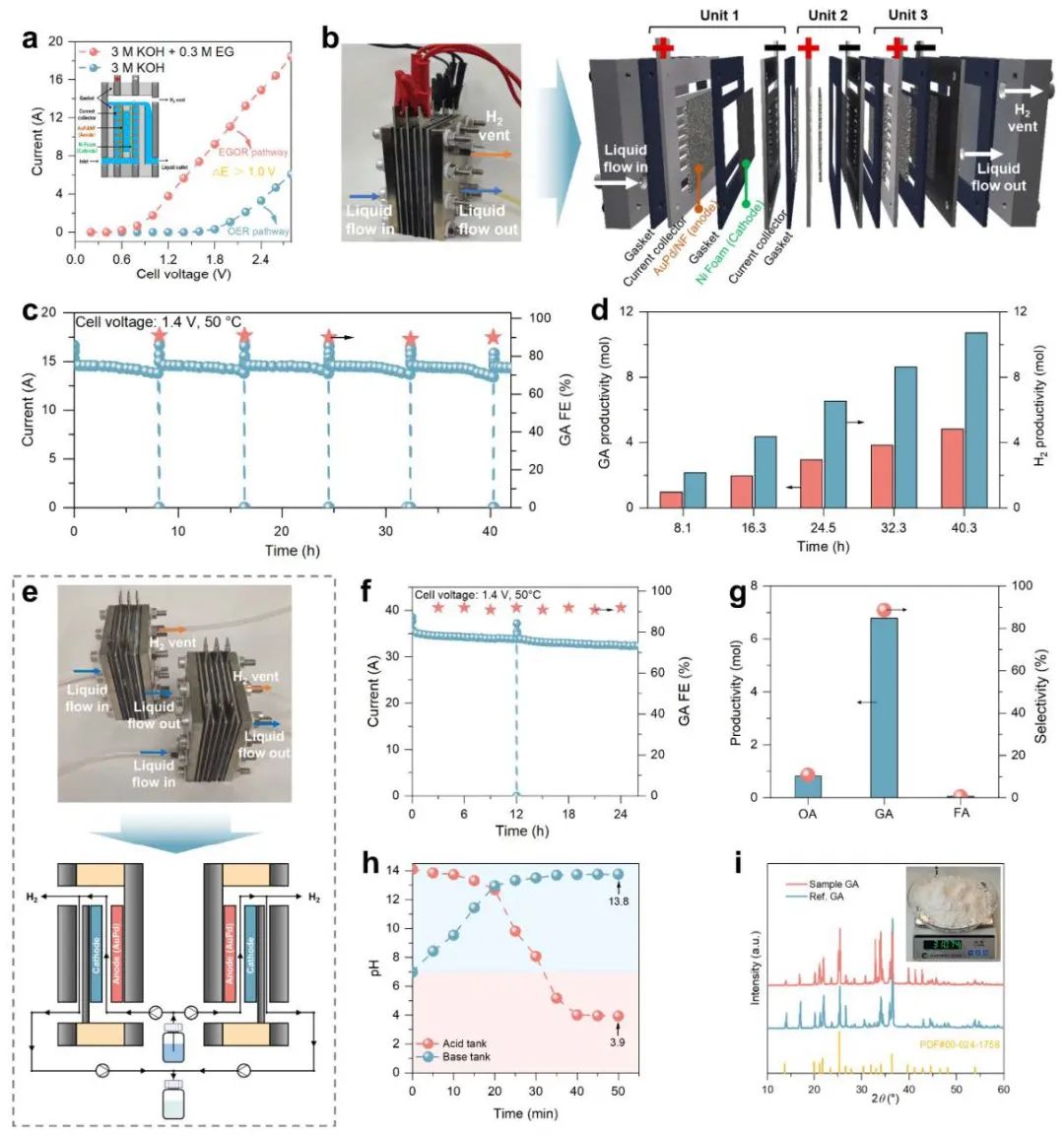
Fig. 3. Device enlargement
Based on the above study, a full-flow electrocatalytic upgrading pathway for waste PET was further proposed, including four key steps, namely alkali hydrolysis, electrocatalytic oxidation, electrodialysis separation and polymerization, which resulted in the conversion of bio-unbiodegradable PET into biodegradable PGA and hydrogen. As a proof-of-concept, 25.1 g of EG was first obtained from 90 g of PET, and 20.9 g of GA and 14.9 L of H2 were finally produced and successfully polymerized into high-purity PGA crystals, which can be subsequently processed into plastic filaments. Preliminary techno-economic analyses showed that the process has significant economic benefits and potential for industrial applications.
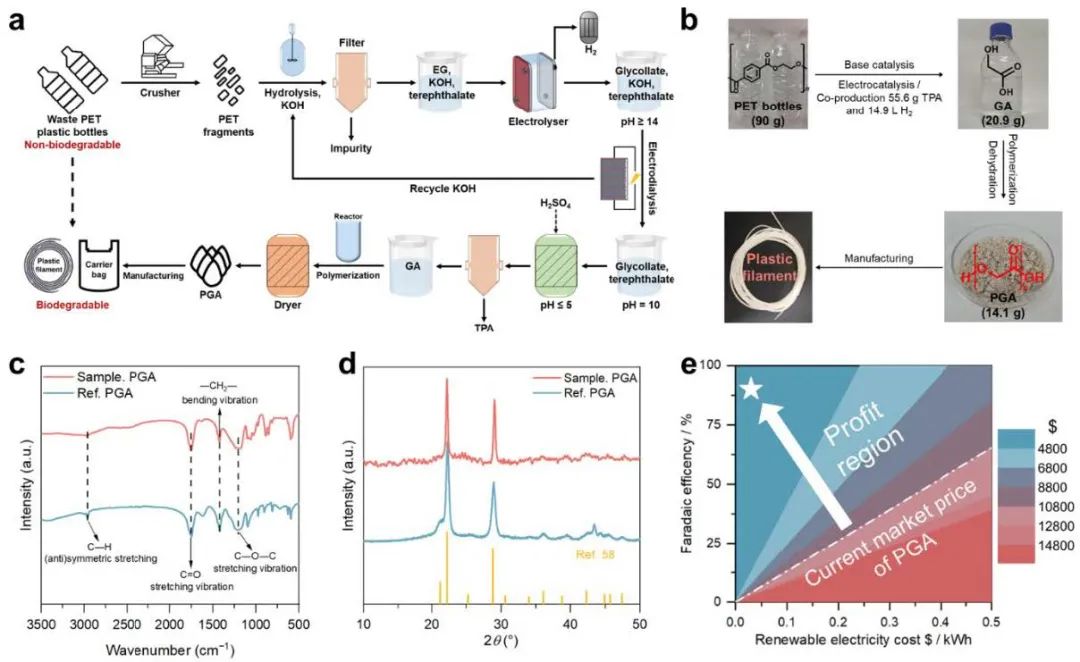
Figure 4. Full Process Transformation
Summary and Outlook:
In summary, this study constructed an efficient and sustainable full-process electrocatalytic upgrading pathway for waste PET, and successfully converted biodegradable PET waste into high value-added biodegradable polymer PGA and clean hydrogen fuel. The developed AuPd alloy catalyst exhibits excellent ethylene glycol electrooxidation activity and long-term stability at amperometric current densities, while the introduction of electrodialysis technology effectively solves the problems of product separation difficulty and alkali waste in the traditional system. This study not only provides a practical green solution for PET pollution management, but also provides a new idea for the integration of electrocatalysis and polymerization reaction, which shows a broad application prospect in the synergistic use of plastic recycling and green energy.
This work was published in CCS Chemistry as Research Article, with graduate students Yan Yifan, Fu Yu and Yang Jiangrong from the School of Chemistry, Beijing University of Chemical Technology (BUCT) as the co-first authors, and Prof. Li Zhenhua and Prof. Shao Mingfei from the School of Chemistry, BUCT as the co-corresponding authors.
