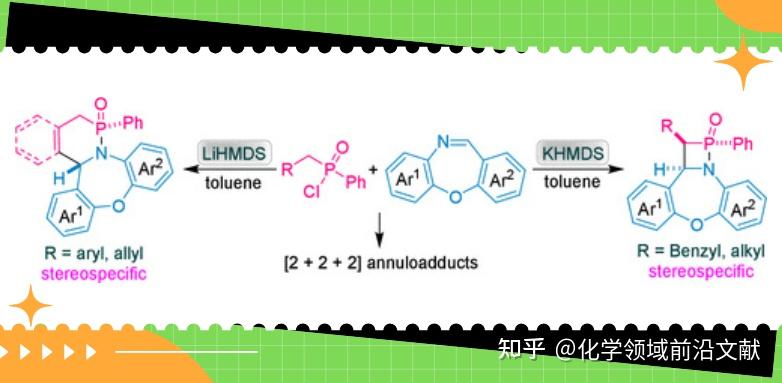
Introduction:
Phosphonolactams with nitrogen-phosphorus heterocyclic structures have important biological activities. For example, cyclophosphonamides and their derivatives containing δ-phosphonolactam backbone have been proved to be effective antitumor drugs; β-phosphonolactam compounds as phosphorus-containing analogs of β-lactam, the key structural part of antibiotics, are expected to be explored as new antibiotics to deal with super-resistant bacteria and so on. The Staudinger cycloaddition reaction of an acyl chloride with an imine in the presence of an organic base produces the antibiotic backbone structure β-lactam; in some cases, two molecules of the enone or imine undergo two different [2+2+2] cyclization reactions. The cyclization reaction of phosphinic chloride, a phosphorus-containing analog of phosphinic chloride, with imines is an important way to explore the phosphine-Staudinger reaction, and provides a new approach for the synthesis of phosphonolactam derivatives. Recently, Jiaxi Xu's group at Beijing University of Chemical Technology reported that different alkyl(phenyl)phosphinic acid chlorides underwent diverse cyclization reactions with imines in the presence of strong bases LiHMDS/KHMDS, and synthesized δ-hypophosphonolactam, β-hypophosphonolactam, and [1, 2, 4]azodiphosphinic acid cyclohexyl derivatives in good yields and in a diastereospecific manner. The reaction requires only 10 min at 40°C in the presence of a base, and is characterized by catalyst-free, mild and rapid reaction conditions. The results were published online in Org. Lett. (DOI: 10.1021/acs.orglett. 4c04737).
Cutting-edge scientific research results:
Diversity cyclization of alkyl(phenyl)phosphinic chloride with imines
The Staudinger cycloaddition reaction between the chloride and imine in the presence of an organic base generates β-lactams with a wide range of antibiotic backbone structures. The chloride first generates an enone in the presence of a base and then undergoes a [2+2] cyclization reaction with the imine. In some cases, two molecules of either the alkenone or the imine undergo two different [2+2+2] cyclizations (DOI: 10.1021/jo302216d). The [2+2] cyclization of allylsulfones with imines, where the allylsulfone undergoes [2+2] cyclization to β-sulfolactam only with N-alkyl imines or with electron-deficient imines in the presence of a strong nucleophilic reagent; similarly to the allyketones, the allylsulfone generated from sulfonyl chloride undergoes in some cases two different [2+2+2] cyclizations with strong nucleophilic imines (DOI: 10.1039/ C5RA15717J). However, one of the phosphorus-containing analogs of the chloride, alkylphosphinic acid chloride, undergoes only hydrophosphonylation with imines in the presence of strong bases to give the corresponding α,β-unsaturated phosphoramidites (Fig. 1a) (DOI: 10.1039/D1QO01949J). In contrast, the reaction of arylmethylphosphinic chloride with imine generated a less diastereoselective cyclization reaction to give the benzo-delta-phosphonolactam (Figure 1b) (DOI: 10.1039/D2NJ05558A). It can be seen that the phosphinic chloride exhibits a completely different chemical reactivity in the reaction with imines than the corresponding chlorides and sulfonyl chlorides. In recent years, Xu Jiaxi's group realized the [2+2] cyclization reaction initiated by the Wolff rearrangement of (diaryl)(aryl)diazomethylphosphine oxides under microwave conditions with chain imines to prepare β-hypophosphonolactams (DOI: 10.1021/acs.orglett.1c03182), and the reaction with cyclic imines to prepare benzyl-δ-hypophosphonolactams (DOI: 10.1021/acs.orglett.0c02346). In order to explore a new strategy for the synthesis of hypophospholactams, especially β-hypophospholactams, the paper investigated the reactivity of different alkyl (aryl) hypophosphoryl chlorides with imines under the action of strong bases LiHMDS/KHMDS (Fig. 1c). The results of the study provide some useful information for phosphine chemistry.
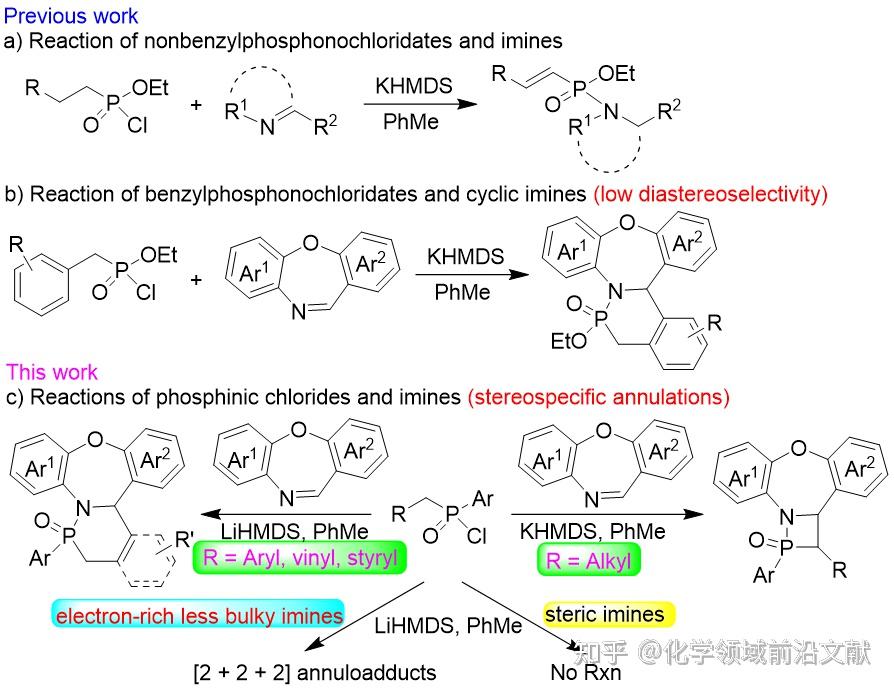
Figure 1. Reaction of imines with phosphinic and phosphinic chlorides
The authors optimized the reaction conditions using benzyl(phenyl)phosphinic chloride (1a) and dibenzo[b,f][1,4]oxazepine (2a) as the raw materials, and the reaction yielded the target products, benzo[b,f][1,4]oxazepine (3aa) and β-phosphinic acid (4aa), in 61 and 25% yields under optimal conditions. In order to examine the substrate applicability of the synthetic method, a series of reactions of alkyl(phenyl)phosphinic chloride 1 containing different substituents with seven-membered cyclic imines and chain imines 2 were attempted. The range of applicability of alkyl phosphinic chloride 1 was first examined (Figure 2).The arylmethyl groups with substituents on the aryl ring or when the benzene ring was replaced with a naphthalene ring as well as with substituents on the benzene ring directly attached to the phosphorus gave the corresponding benzo-delta-hypophospholactams in excellent yields; only the substrates benzyl, 4-chlorophenylmethyl, and phenylethylphosphinic acid chloride yielded the β-hypophospholactams in certain yields (4aa, 4ba, and 4qa), while the other substrates could only be observed in lower yields generation of β-subphosphonolactams. Benzo[b]thiophene-2-methyl(phenyl)phosphinic chloride underwent only a [2+2+2] cyclization reaction to produce 2,4,4a-trihydro[1,2,4]azadiphosphinic acid cyclohexa[1,6-d]dibenzo[b,f][1,4]oxazepine-1,3-dioxide (5na). In addition, allyl(phenyl)hypophosphinic chloride (1o) and cinnamyl(phenyl)hypophosphinic chloride (1p) underwent this reaction to give the corresponding δ-hypophospholactams 3oa and 3pa.
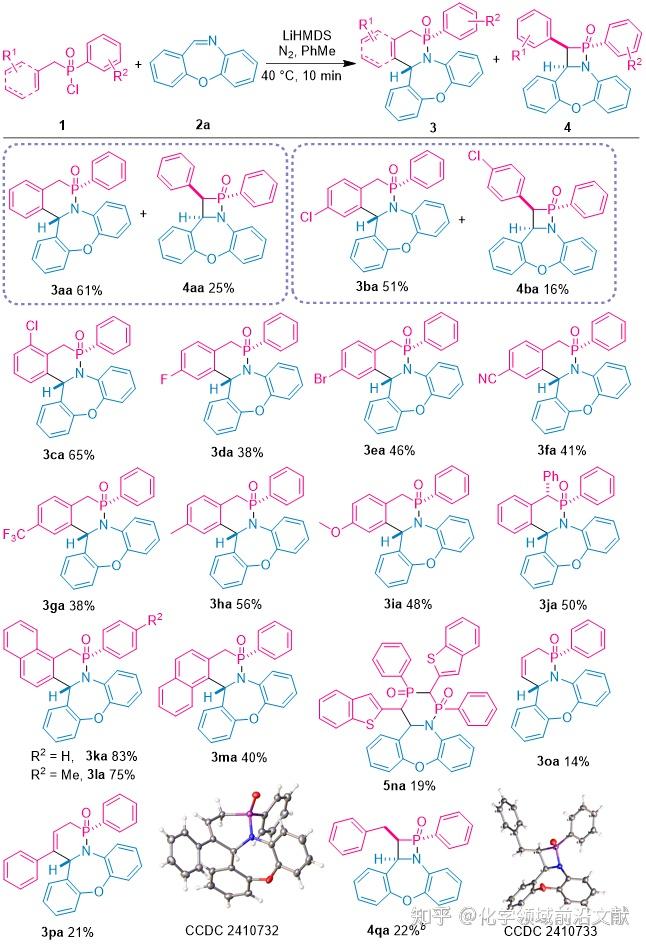
Fig. 2. Study of the range of applicability of the reaction substrates
Subsequently, the range of applicability of imines 2 was examined (Fig. 3). Both seven-membered cyclic imines and α,β-unsaturated imines were synthesized in good yields for the corresponding benzo-δ-phosphonolactams. Substrates 2b and 2i underwent not only [4+2] cyclization but also accompanied by [2+2+2] cyclization to produce the corresponding substrates 5kb and 5ki. cyclic imines with a site-block on the carbon, normal chain imines, as well as the six-membered cycloimines dihydroisoquinoline and phosphinic chloride did not undergo this reaction.
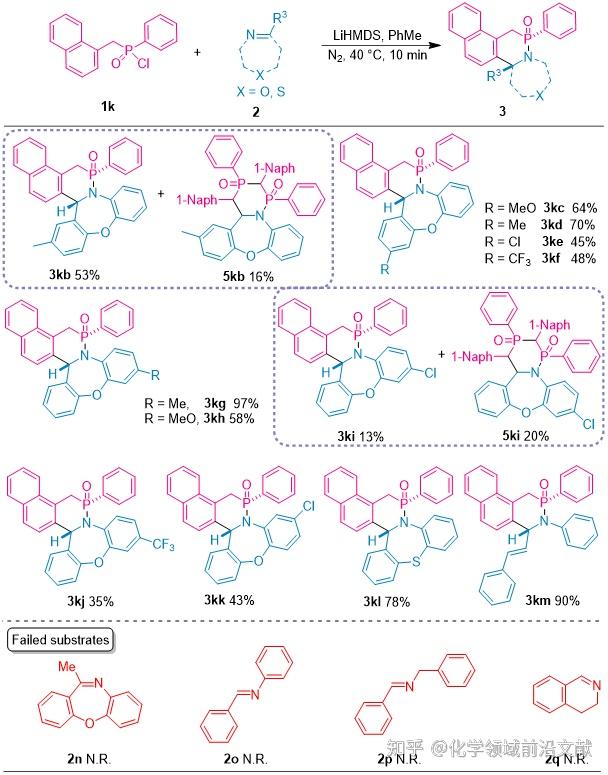
Fig. 3. Study of the range of application of the reacting substrates
