Recently, Professor Mingfei Shao’s research team at Beijing University of Chemical Technology (BUCT) made a significant breakthrough in the field of electrocatalytic plastic recycling and resource utilization. For the first time, the team successfully demonstrated a full-flow electrocatalytic conversion process that transforms polyethylene terephthalate (PET) waste into biodegradable polyglycolic acid (PGA) while co-producing hydrogen gas. Remarkably, the system achieved stable operation for 1,000 hours under ampere-level current densities (exceeding 1 A cm⁻²), marking a critical advancement toward practical industrial applications.
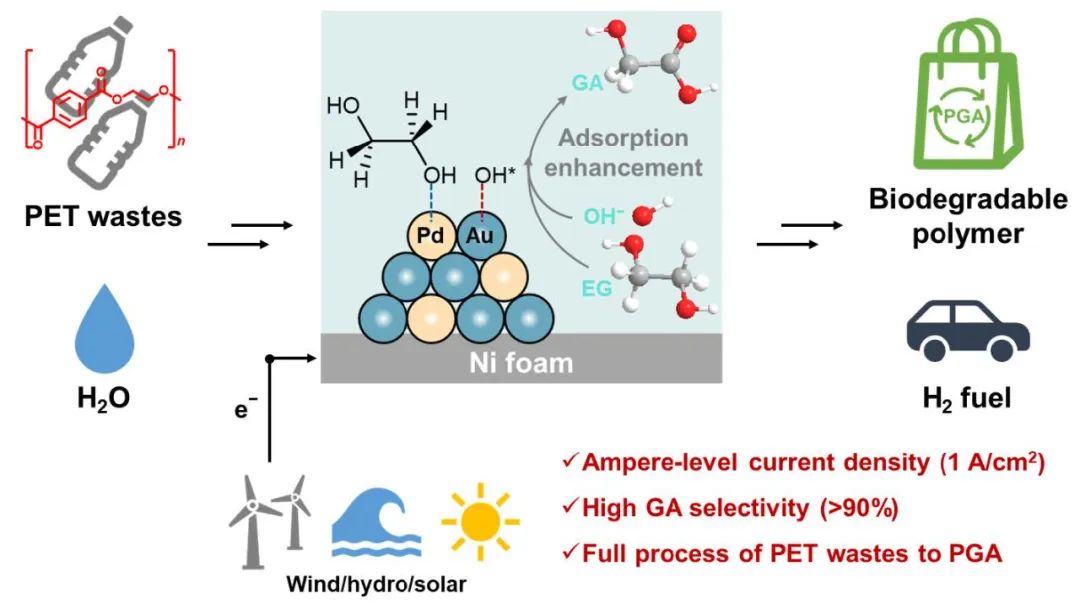
Fig. 1. Conversion of PET waste at high current density to biodegradable PGA and H2
Plastic pollution remains a pressing global environmental crisis, with PET—the most widely used polyester material—contributing significantly to the problem. Annual PET production exceeds 70 million tons, yet less than 20% is recycled. The majority ends up in landfills or oceans, threatening ecosystems and human health. Electrocatalysis has emerged as a promising green technology for plastic upcycling due to its mild reaction conditions, high efficiency, and environmental compatibility. Previous research focused on converting ethylene glycol (EG)—a product of PET hydrolysis—into glycolic acid (GA), a monomer for biodegradable PGA. However, existing systems faced critical limitations: low current densities (far below 1 A cm⁻²), unstable catalysts, and the need for additional acid to neutralize glycolate salts before polymerization. These hurdles increased complexity, cost, and energy consumption, hindering scalability.
To address these challenges, Prof. Shao’s team, leveraging their expertise in electrolytic hydrogen production and coupled oxidation systems, designed an innovative AuPd alloy catalyst with synergistic active sites. In this system, gold promotes the generation of reactive oxygen species (·OH), while palladium enhances the adsorption and enrichment of EG molecules at the catalyst-electrolyte interface. This dual functionality enabled efficient EG electrooxidation at unprecedented ampere-level current densities (>1 A cm⁻²), achieving 94% selectivity for GA and stable operation for 1,000 hours—far surpassing existing catalytic systems.
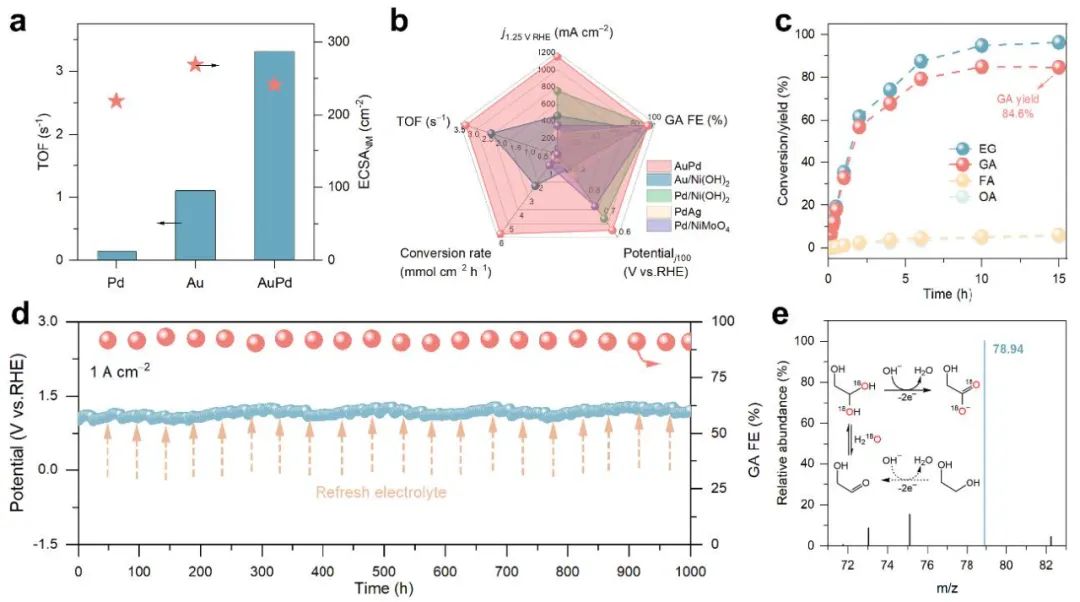
Fig. 2 The catalyst’s performance under high-current-density conditions
The team further scaled up the technology by constructing a diaphragm-free flow electrolyzer, which achieved an impressive oxidation current of 35.1 A under simulated industrial conditions (cell voltage: 1.4 V). This setup produced 515.4 g of GA per day alongside 14.3 L of hydrogen per hour, demonstrating dual outputs of high-value chemicals and clean energy. To streamline the process, electrodialysis was introduced to separate GA products efficiently while recovering 97% of the potassium hydroxide (KOH) electrolyte, eliminating the need for acid neutralization. Coupled with rotary evaporation, high-purity GA crystals (95% selectivity) were obtained, ready for direct polymerization into PGA.
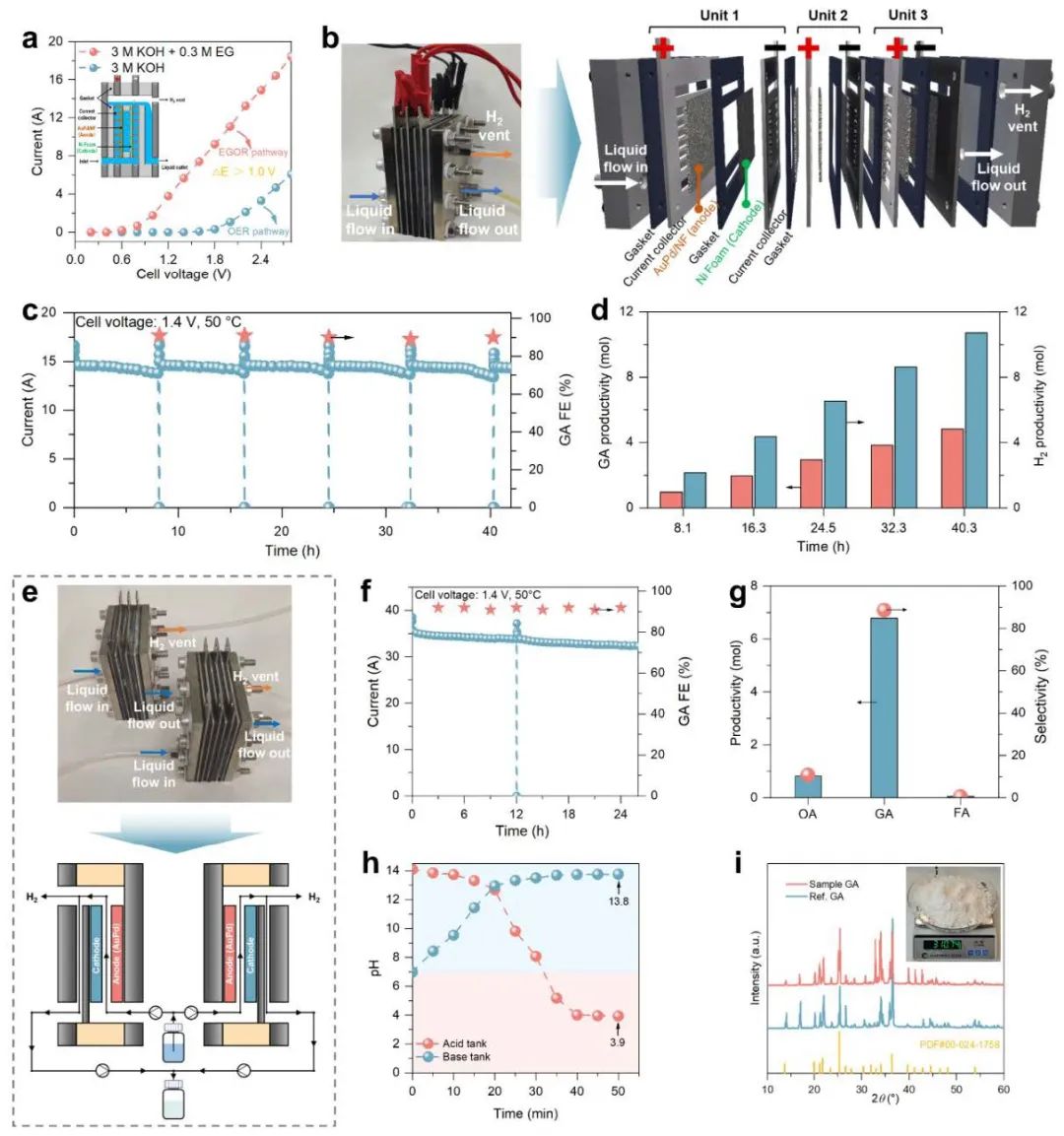
Fig. 3 Highlights the scaled-up reactor and separation system
The researchers validated a closed-loop, full-process pathway for PET upcycling, encompassing four stages: alkaline hydrolysis, electrocatalytic oxidation, electrodialysis separation, and polymerization. Using 90 g of PET waste, the process yielded 25.1 g of EG, which was subsequently converted into 20.9 g of GA and 14.9 L of hydrogen. The GA was polymerized into high-purity PGA crystals, which can be processed into biodegradable plastic filaments. Preliminary techno-economic analysis revealed substantial cost savings and industrial feasibility compared to conventional methods.
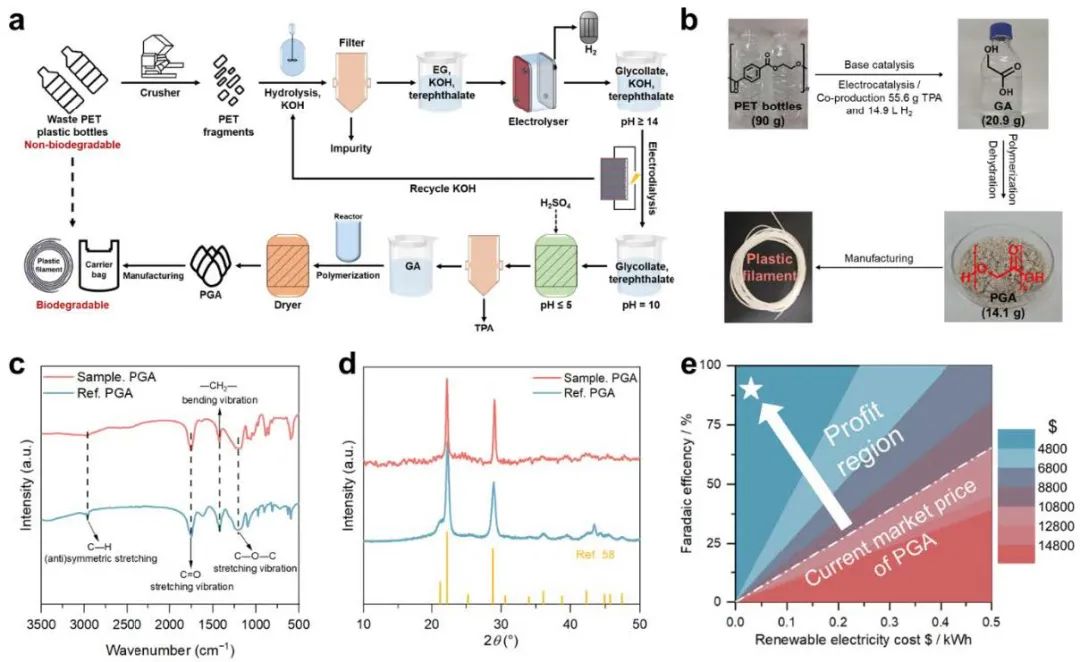
Fig. 4 Outlines the end-to-end conversion process
This study provides a transformative solution for PET waste management. By integrating efficient electrocatalysis with innovative separation and recovery technologies, the team not only addresses plastic pollution but also advances the synergy between sustainable material production and green energy generation. The work establishes a scalable model for converting non-biodegradable plastics into high-value biodegradable polymers and clean hydrogen fuel, offering a blueprint for circular economy innovations.
