A collaborative team led by Prof. He Jing from the School of Chemistry at Beijing University of Chemical Technology, Prof. Duan Xuezhi from the School of Chemical Engineering at East China University of Science and Technology (ECUST), and Distinguished Researcher Chen Wenyao from the National Key Laboratory of Chemical Engineering and Low-Carbon Technology has developed an innovative dual-switch tandem regulation strategy. Their breakthrough work on the Ni@CMO heterojunction catalyst enables the on-demand synthesis of primary, secondary, and tertiary aromatic amines with exceptional yields exceeding 96%. The findings were published in Angewandte Chemie International Edition under the title Tandem Switch-Triggered On-Demand Synthesis of Aromatic Amines in High Yields (DOI: 10.1002/anie.202424847).
Aromatic amines serve as critical intermediates in pharmaceuticals, dyes, agrochemicals, rubber additives, and heterocyclic compounds. Conventional synthesis methods, however, suffer from multi-step processes, low atom economy, harsh conditions, excessive byproducts, and environmental concerns. While one-pot tandem catalysis offers a greener alternative by integrating reactions in a single reactor, challenges persist due to complex mechanisms, competing pathways, intermediate incompatibility, and the difficulty of designing catalysts with three or more active sites without mutual inhibition.
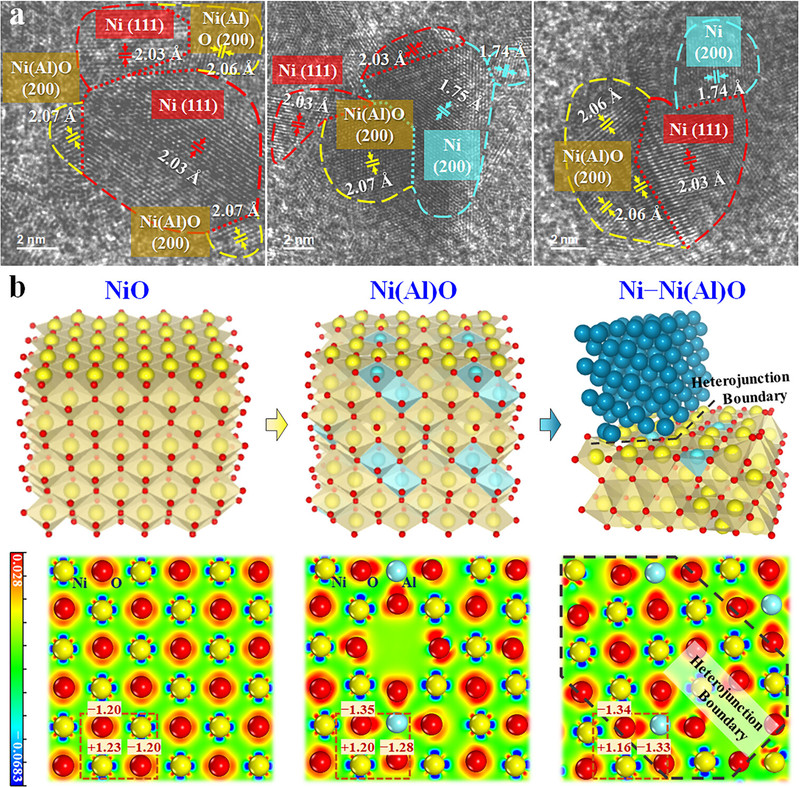
The team’s kinetic-thermodynamic dual-switch strategy systematically addresses incompatibility issues in multi-step reactions. The kinetic switch leverages a phosphotungstic acid-functionalized Ni–Ni(Al)O heterojunction catalyst to precisely regulate metal-metal and metal-oxygen microenvironments, accelerating rate-determining steps in nitrobenzene hydrogenation, N-alkylation, and aza-Michael addition. The thermodynamic switch employs competitive adsorption of ethanol, hydrogen, and acrylonitrile to minimize free energy changes, ensuring smooth cascade reactions. This approach enables in situ pathway switching and on-demand synthesis of mono-, di-, and tri-substituted aromatic amines with >96% yields, while circumventing traditional reliance on site isolation or protection.

The Ni@CMO catalyst, crafted through in situ topological transformation, features abundant Ni–Ni(Al)O heterojunction boundaries and a tunable metal/non-metal acid-base synergy. Integrated mesoscopic kinetic studies (isotope tracing, kinetic analysis, and DFT calculations) elucidated rate-determining steps and critical active sites for each substep, establishing a structure-performance relationship between interfacial electronic states and catalytic activity. This system simplifies aromatic amine production, reduces energy/material consumption, and enhances process sustainability and cost efficiency.
The study was co-first authored by Chen Zhou and Sang Keng (ECUST doctoral candidates), Ye Lei (postdoctoral fellow), and Assoc. Prof. Zhang Jian (Beijing University of Chemical Technology). Academicians Yuan Weikang and Chen De, along with Prof. Zhou Xinggui, provided guidance. Support came from the National Key R&D Program, National Natural Science Foundation, Shanghai Carbon Neutrality Basic Research Special Zone Project, Shanghai Municipal Education Commission’s Scientific Research Innovation Program, and Shanghai Science and Technology Commission’s Innovation Action Plan.
Original Article:
https://onlinelibrary.wiley.com/doi/abs/10.1002/anie.202424847
