Background
The utilization of biomass resources as an alternative to fossil resources for the preparation of fuels and chemicals has become a hot topic of research today. The effective utilization of ethanol, an important biomass platform molecule, is crucial for the conversion of fibrous biomass. In recent years, the bioethanol industry has been booming globally, and the high-value conversion of ethanol to fine chemicals has become the key to promote the development of the downstream industry chain of ethanol. However, due to the diversity of chemical bond types in biomass resources molecules, the conversion to high-value-added chemicals faces many challenges, such as poor atom economy and low degree of greening. Among them, the efficient activation and targeted conversion of oxygen-related chemical bonds has become a central scientific issue.
Acetal, as a multifunctional high-value fine chemical, has a wide range of applications in many fields such as medicine, energy, chemical and food. Acetal is usually prepared industrially by the condensation reaction between ethanol and acetaldehyde, but the toxicity and non-storable nature of acetaldehyde make it particularly important to find an efficient conversion pathway with ethanol as a single reactant. However, from a thermodynamic point of view, the oxidative dehydrogenation of ethanol for the preparation of acetaldehyde requires high temperatures to activate oxygen and efficiently break the α-C-H bond of ethanol, whereas the subsequent condensation reaction of the aldehyde with ethanol is a reversible and exothermic reaction, and low temperatures are more favorable for efficient acetalization. This mismatch of the two-step tandem catalytic reaction conditions makes the efficient preparation of acetal in one step with high conversion and selectivity a great challenge.
Brief description of results
In this study, a Bi/BiCeOx bifunctional nanocatalyst with strong interfacial interactions was designed and prepared. Under the conditions of atmospheric pressure, 150 °C, and air oxidation, the catalyst realized the efficient conversion of ethanol by oxidative dehydrogenation and acetalization in tandem in a one-pot method, and acetal was successfully prepared. The experimental results showed that the conversion of ethanol was as high as 87.0±1.0%, while the selectivity of acetalization reached 98.5±0.5%. It was further shown that the Biδ+-Ov-CeIII sites at the Bi/BiCeOx interface were the key to realize efficient tandem catalysis. Among them, the Biδ+-Ov-site can effectively promote the oxidative dehydrogenation of ethanol under milder conditions, while the -Ov-CeIII site contributes to the efficient acetalization reaction of acetaldehyde intermediates with ethanol.
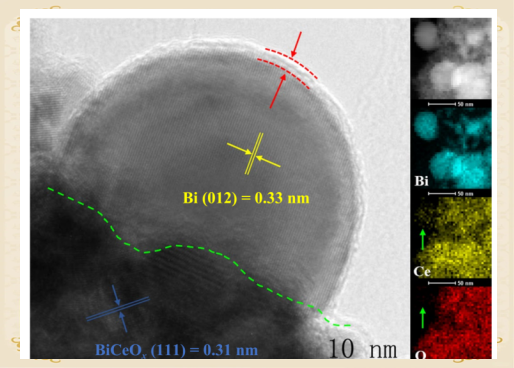
Figure 1 illustrates the fine interfacial structure of Bi/BiCeOx nanofunctional catalysts with strong interfacial interactions.
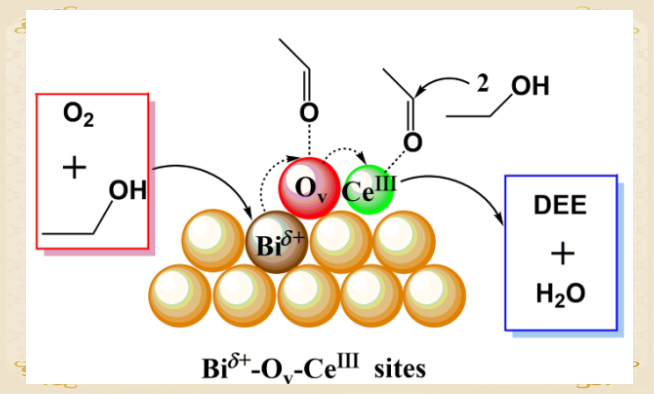
Figure 2 demonstrates a possible mechanism for the efficient preparation of acetal by the one-pot method of ethanol catalyzed by nano-Bi/BiCeOx with strong interfacial interactions. This research was led by He Jing, a professor at Beijing University of Chemical Technology, whose team has made significant progress in the field of nanocatalysis and effective utilization of biomass resources. The relevant results have been published in Nano Research and other well-known journals.
