On March 6, the team led by Xiaoming Sun and Daojin Zhou from our college collaborated with Professor Bin Liu's team at City University of Hong Kong to reveal issues such as oxidation and corrosion in hydrogen-producing cathodes during the (sea)water electrolysis process coupled with fluctuating renewable energy. Based on this, they proposed the in-situ construction of a multi-layer passivation structure to resist oxygen penetration in cathodes under shutdown conditions, providing a new solution to the bottleneck problem of alkaline electrolyzers and anion exchange membrane electrolyzers coupled with fluctuating energy. Related achievements were published in Nature under the title “10,000-hour stable intermittent alkaline seawater electrolysis”. Qihao Sha, a 2022 master's student at Beijing University of Chemical Technology, and Shiyuan Wang, a graduated doctoral student, are the co-first authors of this paper, with Beijing University of Chemical Technology as the first completion unit.
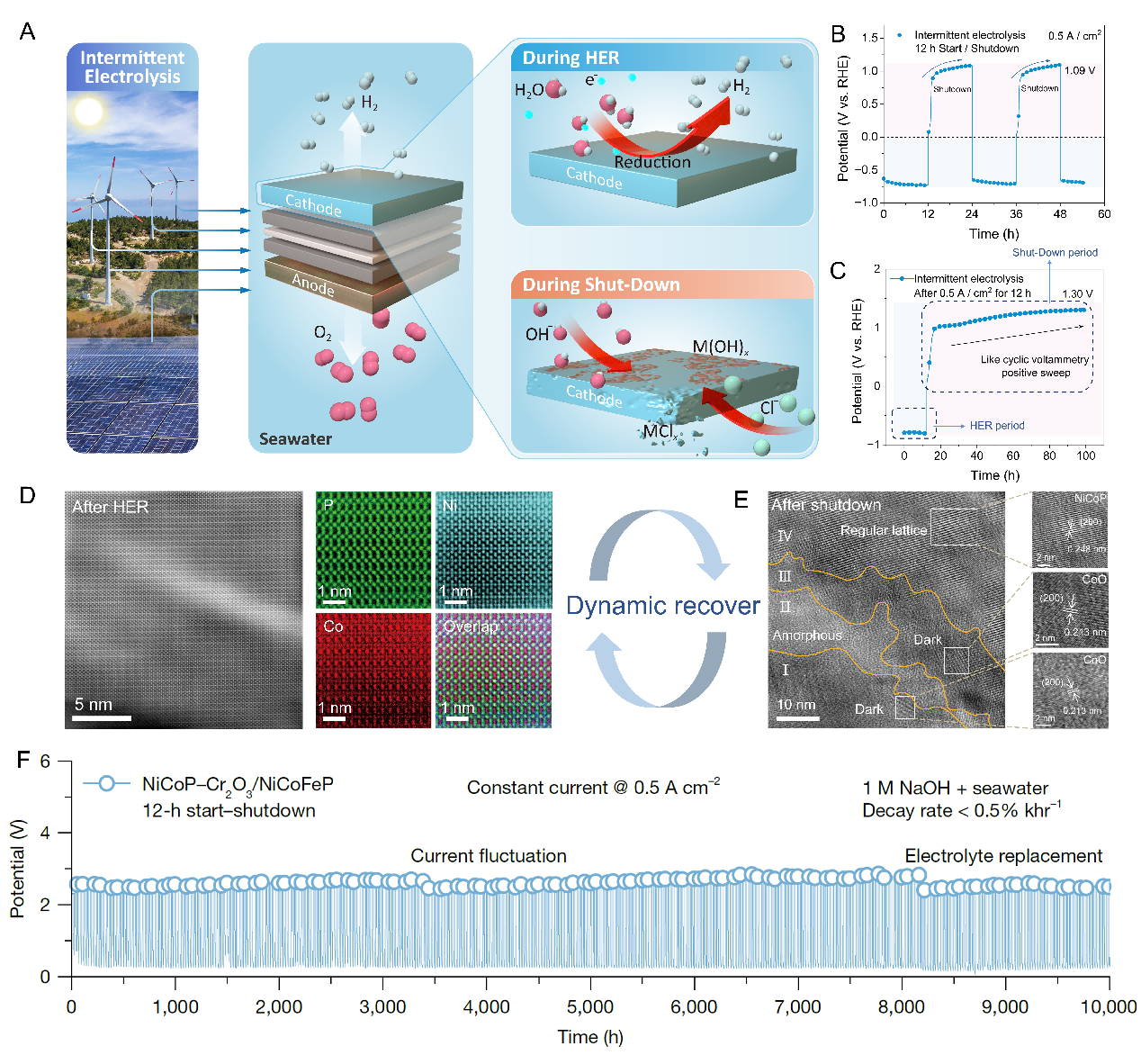
Figure Schematic diagram of cathode oxidation and corrosion during intermittent water/seawater electrolysis cycles (A); quantitative analysis of cathode oxidation potential under shutdown conditions (B, C); aberration-corrected electron microscopy images before and after start-stop cycles (D, E); and stability test of electrode intermittent electrolysis (F).
Electrolysis of water driven by renewable energy (green electricity) is regarded as a crucial approach for sustainable production of "green hydrogen" and achieving the dual-carbon goals. However, the current mainstream alkaline water electrolysis technology faces challenges of poor stability and difficulty in integrating with renewable energy. Renewable energy is characterized by intermittency, volatility, and randomness. The research team found that the water electrolysis cathode coupled with fluctuating renewable energy experiences reverse current under shutdown conditions, which can raise the cathode potential to 1.09 V (vs. RHE) within 12 hours of shutdown. This exposes the metallic cathode catalyst materials to excessive oxidation by OH⁻ in the electrolyte. When seawater is used as the feedwater, the large amount of halide ions (Cl⁻, Br⁻) in seawater will pose a more severe corrosion challenge to the cathode under shutdown conditions.
In response to the above challenges, the research team synthesized a NiCoP-Cr₂O₃ hydrogen evolution catalyst electrode based on a metal phosphide nanoarray covered with an oxide layer, and revealed the dynamic reconstruction process of the hydrogen evolution electrode during the start-stop operation of seawater electrolysis. During the shutdown process, a multi-layer passivation layer composed of cobalt oxide - cobalt phosphate - cobalt oxide is in-situ generated, which effectively resists oxygen penetration during the shutdown process and prevents excessive oxidation of active Ni by OH⁻, enabling the active sites to be reactivated when the next hydrogen evolution cycle starts. This improves the recyclability and stability of the catalytic active centers under current/voltage fluctuation conditions. Electrodes designed with this strategy can operate intermittently in alkaline seawater at an industrial current density of 0.5 A cm⁻² for 10,000 hours, with a voltage increase rate of only 0.5% khr⁻¹. Even when operating intermittently at an ultra-high current of 10 A cm⁻², it still maintains good stability (1.8% khr⁻¹ @ 700 h).
This work proposes a new passivation mechanism and material design strategy, providing new ideas for solving the problem of performance degradation of hydrogen evolution electrocatalysts during fluctuating electrolysis processes, and offering key technical support for promoting the industrialization of (sea)water electrolysis technology driven by renewable electricity.
Original link: https://www.nature.com/articles/s41586-025-08610-1
Introduction to Authors:

Qihao Sha, a 2022 master’s student at Beijing University of Chemical Technology (supervised by Professor Xiaoming Sun). He obtained his bachelor’s degree from Beijing University of Chemical Technology between 2018 and 2022 (supervised by Professor Xiaoming Sun). His main research directions include hydrogen production via (sea)water electrolysis and operando spectroscopy characterization. He has published 6 papers as the first author (including co-first authors) or corresponding author in journals such as Nature and Nature Communications, and has applied for 8 national invention patents and 1 international patent. He has received honors such as "Outstanding Graduate of Beijing" and "Outstanding Undergraduate Thesis of Beijing".

First Author:
Shiyuan Wang, currently an engineer at State Power Investment Corporation Hydrogen Energy Tech Co., Ltd. He received his Bachelor of Science and Doctor of Science degrees from Beijing University of Chemical Technology in 2017 and 2022, respectively (supervised by Professor Xiaoming Sun). His main research areas include the development of high-efficiency materials for hydrogen production via (sea)water electrolysis and key materials for high-performance fuel cells. He has published 5 papers as the first author (including co-first authors) in journals such as Nature and Research, and has applied for 8 national invention patents.

Corresponding Author
Daojin Zhou, Associate Professor at Beijing University of Chemical Technology. He completed his PhD at Beijing University of Chemical Technology in December 2019, followed by postdoctoral research at the University of Toronto and Northwestern University. His research interests focus on inorganic material synthesis and electrocatalytic applications. He has published over 40 papers as (co-)first author or corresponding author in internationally renowned journals such as Nature, Nature Communications, Angewandte Chemie International Edition, Chemical Society Reviews, and Science Bulletin, with over 4,000 citations. As a project leader, he has presided over 7 projects, including the General Program and Young Scientists Fund of the National Natural Science Foundation of China, and the Young Scientists Program of the Beijing Natural Science Foundation. In 2022, he was supported by the 8th Young Elite Scientists Sponsorship Program (2022–2024) of the China Association for Science and Technology.
Corresponding Author
Xiaoming Sun, Professor and PhD Supervisor at Beijing University of Chemical Technology. He received his Bachelor of Science and Doctor of Science degrees in Chemistry from Tsinghua University in 2000 and 2005, respectively. After completing postdoctoral research at Stanford University in 2008, he returned to China and joined the State Key Laboratory of Chemical Resource Engineering at Beijing University of Chemical Technology. In 2011, he was funded by the National Science Fund for Distinguished Young Scholars, and in 2019, he was recognized as a leading talent in the Ten Thousand Talents Program of the Central Organization Department.
His research focuses on inorganic nanomaterial chemistry, with notable advancements in water electrolysis, fuel cells, and gas-superwettable electrode devices. He has published over 200 papers as a corresponding author in leading international journals of energy materials and chemistry, including Nature, Nature Catalysis, Joule, Chem, Nature Communications, Proceedings of the National Academy of Sciences of the United States of America, Journal of the American Chemical Society, Angewandte Chemie International Edition, Advanced Materials, Chemical Society Reviews, and Accounts of Chemical Research, with over 30,000 citations. He has also authored one academic monograph.
Dr. Sun has applied for 13 international patents (3 granted) and obtained over 80 authorized national invention patents, with nearly 10 successfully commercialized. He has led multiple major research projects, including key programs of the National Natural Science Foundation of China, international (regional) key collaborative projects, and national key research and development projects.

