
Research background
As a sustainable method, electrolysis technology can be driven by renewable energy such as solar energy and wind energy to achieve high value conversion of waste PET plastics and CO ₂ under mild conditions (such as production of formate). At present, significant progress has been made in the development of catalytic materials and reaction systems, but there are obvious bottlenecks in the existing technology: the reaction is usually carried out under alkaline conditions, and subsequent acidification separation steps are required. The traditional method requires the introduction of commercial formic acid or sulfuric acid and other product acids, resulting in increased resource consumption and reduced overall sustainability. Therefore, it is of great significance to develop efficient conversion processes that reduce dependence on external resources.
Article Overview
Shao Mingfei and Zhou Hua from Beijing University of Chemical Technology put forward an innovative decoupling electrolysis system to synergistically transform waste PET plastics and CO2 into high-value products - potassium dicarboxylate, terephthalic acid and hydrogen fuel. The core of this technology is to realize step-by-step conversion through two reactors: (1) porous solid electrolyte (SSE) reactor: for CO two Reduction, continuous operation for more than 140 hours under 250 mA current, producing about 850 mL of pure formic acid solution with a concentration of 683 mM, significantly improving the yield and stability; (2) Solid polymer electrolyte (SPE) reactor: it is used for the electrooxidation of ethylene glycol to produce formate, achieving 30 A reaction current at 1.9 V, and the Faraday efficiency of formate reaches 80%. In addition, the team directly electrolyzed PET hydrolysate to obtain formate, terephthalic acid and hydrogen fuel simultaneously. Finally, through coupling design, CO two The derived formic acid solution acidified PET electrolyte, successfully separated potassium dicarboxylate and recovered terephthalic acid, without the need for external acid in the whole process, greatly improving sustainability.
Research contents
In the previous research, the team proposed a series chemical electrochemical process for the high-value utilization of PET plastics, aiming at recycling PTA and producing high-value KDF and hydrogen fuel. However, this process needs to introduce external commercial formic acid to acidify PET electrolyte to precipitate PTA and react with formate to generate KDF. At present, commercial formic acid is mainly produced from fossil materials, which largely affects the sustainability of the whole process. Recently, other research teams proposed PET electrolysis and electrochemical CO two The reduction reaction is combined to produce formic acid to achieve sustainable development. However, this method still needs to use exogenous inorganic acids (such as H two SO four )The value of formic acid obtained by acidification is relatively low. To solve the above problems, this paper proposes the decoupling strategy shown in Figure 1c, using CO two Reduction of in-situ formic acid solution acidifies PET electrolyte without introducing external acid source, so as to realize sustainable production of KDF and hydrogen and effective recovery of PTA.
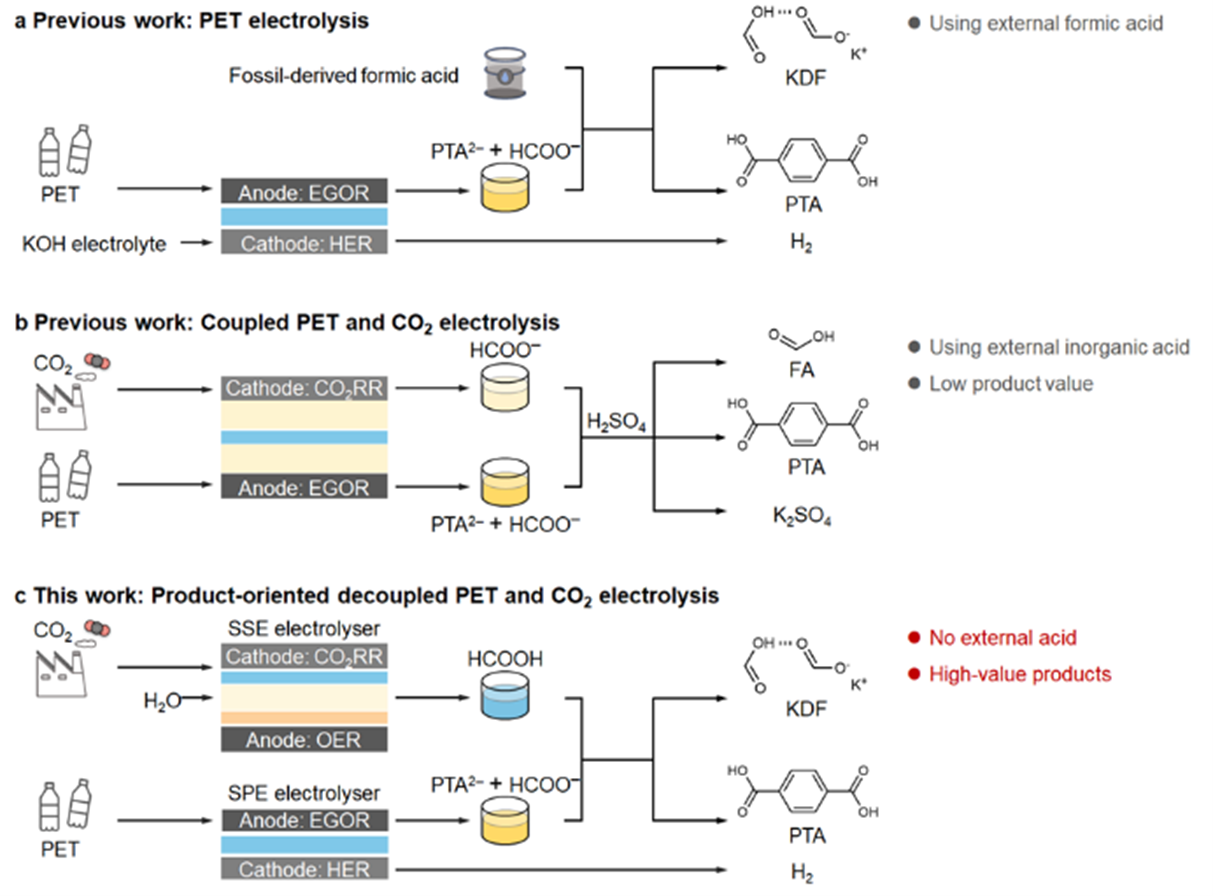
Fig. 1 Design diagram of potassium dicarboxylate synthesis process. a) Preliminary work of PET electrolysis and HER coupling. b) PET electrolysis and CO two Preliminary work of RR coupling. c) This work design: product oriented decoupling PET and CO two electrolysis.
For the screened CO two RR catalyst Bi two O two CO two (BOC) was systematically characterized and its electrochemical performance evaluation showed that BOC presented an irregular nano sheet shape, with a lattice spacing of 3.72 ∨, corresponding to the (011) crystal plane of BOC, and the PDF # (41-1488) card of XRD diffraction peak was highly matched, which confirmed the successful construction of BOC. The electrochemical test results show that BOC has excellent CO2RR formic acid production performance, maintaining>90% formic acid FE in a wide potential range of − 0.9 to − 1.3 V vs RHE; reaching about 60 mA cm at − 1.3 V vs RHE −2 Current density of formic acid and 1.1 mmol cm −2 h −1 Yield of.
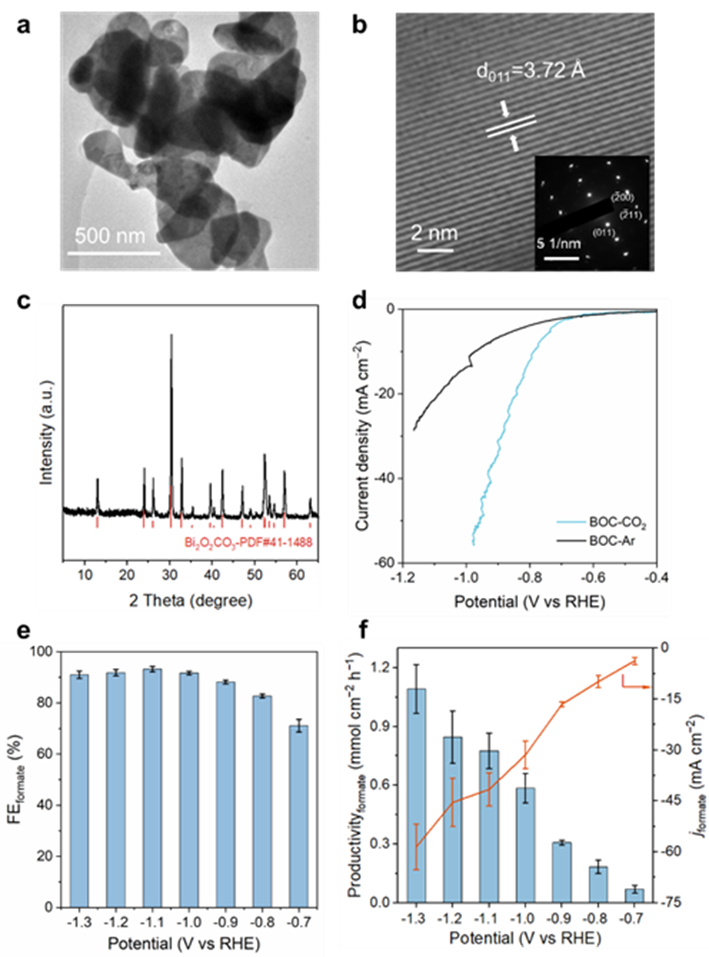
Fig. 2 Characterization of BOC catalyst and CO two RR performance evaluation. a) TEM image. b) High resolution HRTEM images and SAED maps (illustration). c) XRD diagram of BOC catalyst. d) Ar/CO2 saturation - 0.5 M KHCO three In the LSV curve of BOC, the potential is corrected by iR. e. F) e) formate FE, f) formate yield and partial current density obtained under different applied potentials.
With BOC catalyst as cathode IrO two 5 cm for anode assembly two The PSE reactor realizes efficient production of formic acid within the current range of 25-100 mA cm ⁻ ², and the FE is stable at~90%. Under the condition of 50 mA cm ⁻ ², the system runs stably for more than 140 hours and produces~850 mL formic acid solution with a concentration of 0.683 mM. The NMR spectrum confirmed that the liquid product was only formic acid, and there were no other by-products.
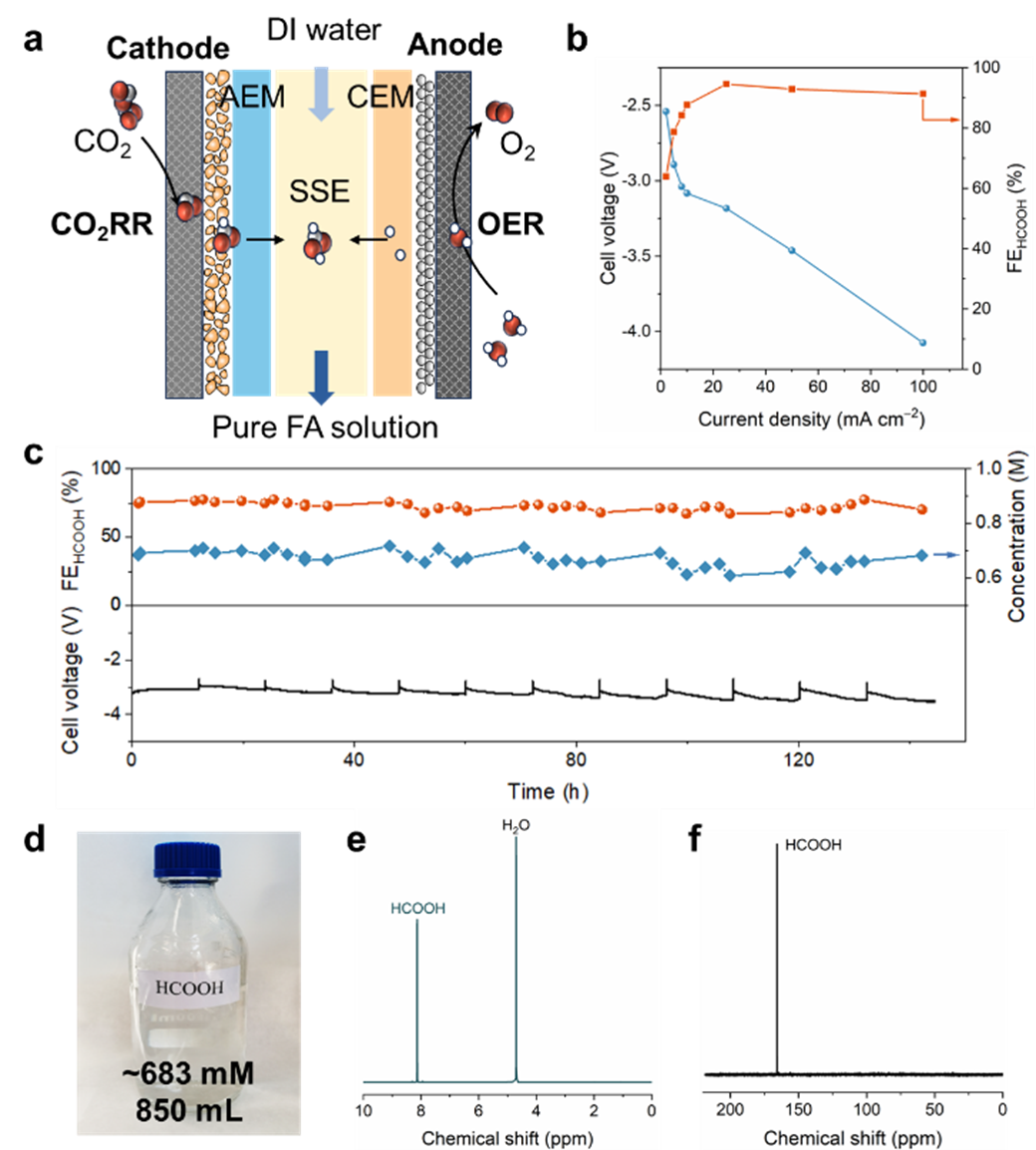
Fig. 3 Production of formic acid aqueous solution in SSE reactor. a) Schematic diagram of SSE reactor. b) Under different current densities, the battery voltage and FE of formic acid produced by BOC catalyst in SSE reactor. c) Under 50 mA cm − 2 current density, the stability test of SSE reactor and the FE and concentration changes of formic acid products in the process. d) Photo of formic acid solution prepared for stability test. e. F) e) 1H NMR and f) 13C NMR tests of the prepared formic acid solution.
For Ni (OH) two /The systematic characterization and electrochemical performance evaluation of NF catalyst showed that Ni (OH) two The nano sheet presents 2.60 ∨ lattice spacing (corresponding to the (012) crystal plane), and its XRD diffraction peak is completely matched with the standard card (PDF # 38-0715), which confirms that β - Ni (OH) two Successful construction of crystal structure. Electrochemical tests showed that the catalyst maintained more than 80% of the product FE in a wide potential window (1.5 – 1.8 V vs RHE), and reached 293.2 mA cm at 1.8 V vs RHE −2 Current density of formic acid and 3.63 mmol cm −2 h −1 Yield of.
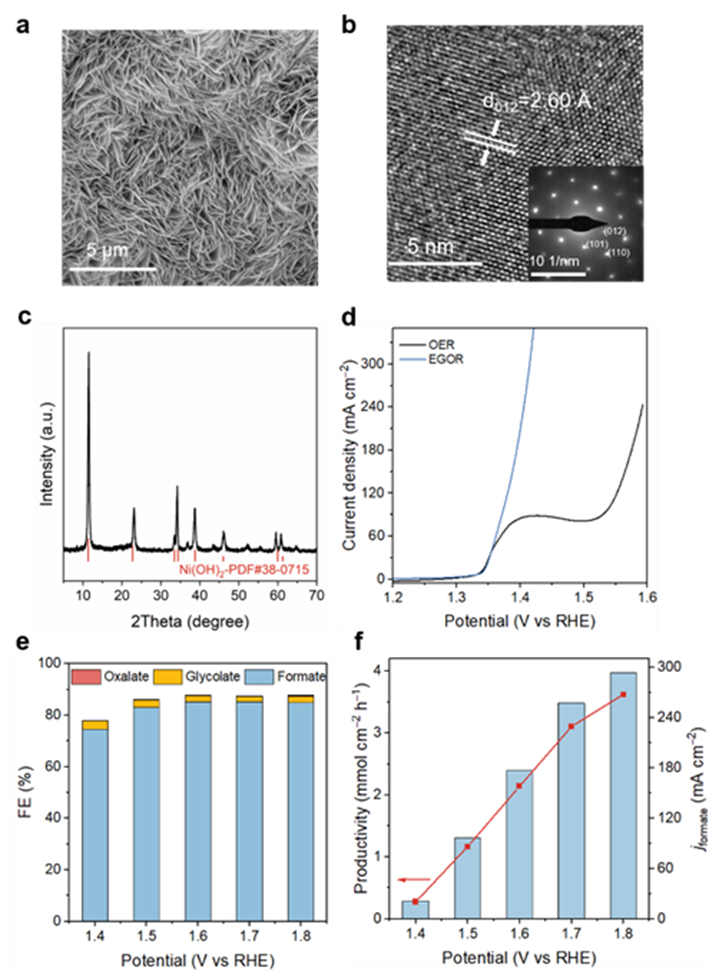
Figure 4 Ni (OH) two Characterization and EGOR performance evaluation of the catalyst. a) SEM image. b) High resolution HRTEM images and SAED maps (illustration). c) Ni(OH) two XRD diagram of catalyst. d) Ni (OH) in 1 M KOH with/without 0.3 M EG two LSV test curve, potential corrected by iR. e. F) e) formate FE, f) formate yield and partial current density at different applied potentials.
50 cm based on self-assembly two SPE reactor, using Ni (OH) two /The large-scale performance of electrooxidation of ethylene glycol (EGOR) to formic acid was systematically investigated in the coupling system of NF anode and RuO2 cathode. Experiments show that at 100 – 600 mA cm −2 Within the wide current density range of, formate FE always maintains at~80%, showing excellent current adaptability. When the total current is increased to 30 A, the reactor can achieve 285.3 mmol h with only 1.9 V of low operating voltage −1 The yield of formic acid is the highest level in the existing literature. The system was further extended to the actual PET hydrolysate electrolysis, and the continuous synthesis of formate was successfully realized. This breakthrough provides key technical support for efficient electrochemical conversion of waste polyester resources.
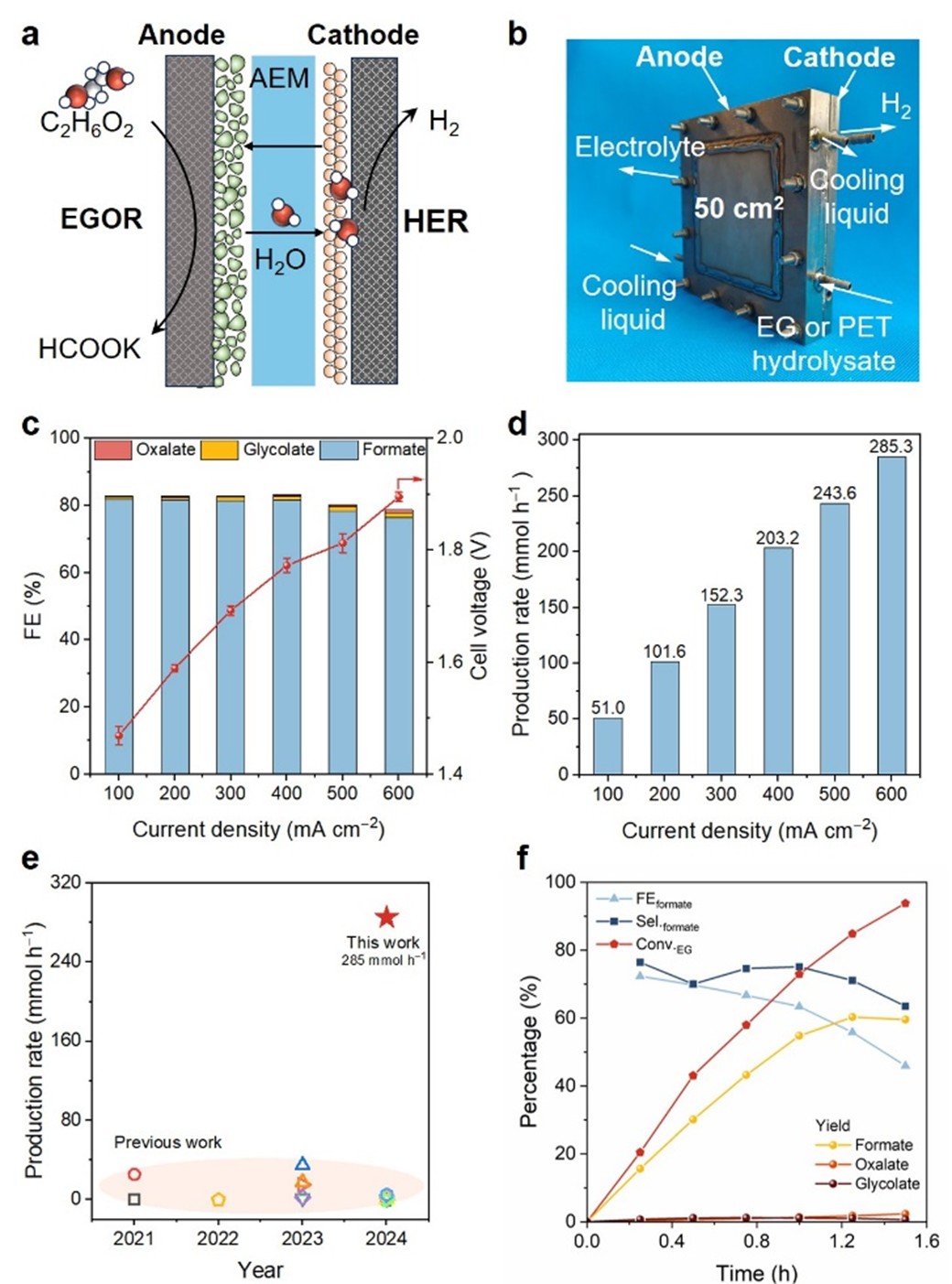
Fig. 5 Formate production in SPE reactor. a) Schematic diagram of SPE reactor. b) Electrode area is 50 cm two Physical photos of SPE reactor. c) FE and battery voltage of the product at different current densities. d) The yield of formate in SPE reactor at different current densities. e) This study is compared with previous studies on the yield of formate produced by EGOR. f) Catalytic performance of circulating electrolysis of PET hydrolysate in SPE reactor under 5A current.
The PET electrolyte obtained by SPE reactor was acidified with formic acid solution produced by PSE reactor, realizing the production of KDF and the recovery of PTA. The whole process does not require the introduction of exogenous acid to achieve the renewable and sustainable development of all raw materials.

Fig. 6 Experimental process diagram of PET and CO2 electrolytic upgrading and conversion process.
Conclusion
In this paper, an innovative decoupling electrolysis strategy is proposed to synchronously convert waste PET plastic and CO2 into high value-added products KDF, PTA and hydrogen fuel by coupling a dual reactor system. Specifically, a small PSE reactor (5 cm two ), continuously running for more than 140 hours under 250 mA current, efficiently producing about 850 mL of 683 mM formic acid solution; Meanwhile, large size SPE reactor (50 cm two ), achieving a breakthrough yield (285 mmol h) of ethylene glycol electrooxidation to formic acid at 30 A total current −1 )And simultaneously convert PET hydrolysate into formate. Finally, CO two Derived formic acid was used as acidifier of PET electrolyte, KDF was successfully separated and PTA was recovered. This work is the first to build a new decoupling electrolytic conversion strategy for waste carbon resources (PET, CO two )Its modular reactor design and product co production mechanism provide a new paradigm for green electrochemical synthesis technology, which is expected to promote chemical production to replace non renewable resources.
