
In alkaline HER processes, beyond the localized structure on the catalyst surface is a dynamic boundary on the electrode - a localized interfacial structure where the solid catalyst interacts with the surrounding electrolyte, an interface that helps to control electron transfer and proton reduction process kinetics. Manipulation of the catalyst surface structure shows the potential to alter the electrolytic-electrolyte interface. Comprehensive studies of the electrode-electrolyte interface in alkaline HER are limited and have focused on platinum (Pt)-based catalyst systems. On Pt-based catalysts, the electrode-electrolyte interface promotes a delicate balance of water/proton adsorption, electron transfer, and desorption processes, which play a key role in the rate-determining step in alkaline environments. However, due to the high cost and limited availability of Pt, there is an urgent need to explore effective alternative catalyst materials. Recent studies have shown that relatively inexpensive Ru-based electrocatalysts can efficiently catalyze alkaline HER because they have comparable water-binding capacity to Pt. Despite these advantages, studies on the effect of local structural modulation on the catalytic interface and performance of Ru-based catalysts are still limited.
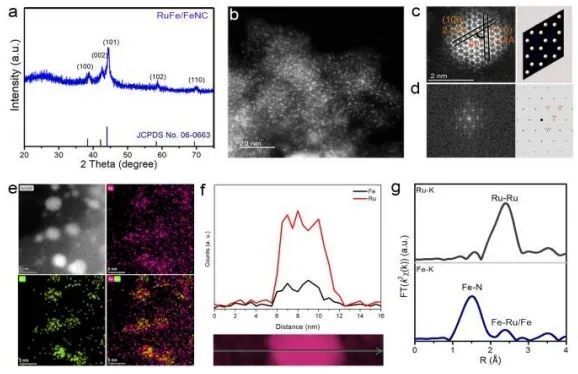
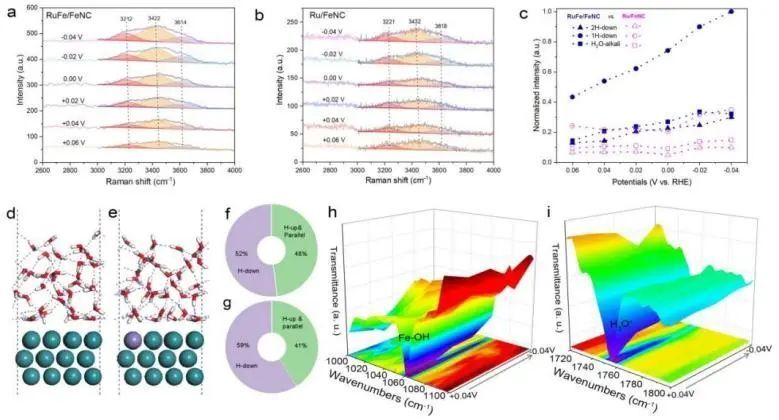
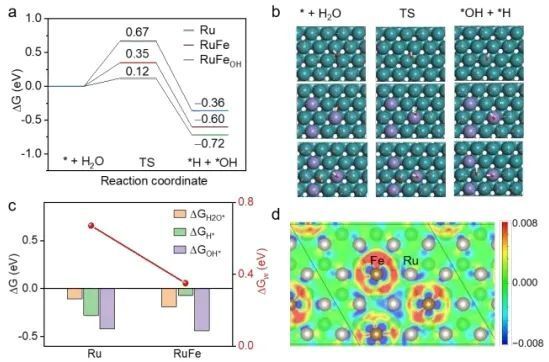
Recently, Song Li, Jiang Jun and Mou Lihui from University of Science and Technology of China (USTC) have synthesized Fe-alloyed defective carbon-loaded RuFe nanoparticles (RuFe/FeNC), which are capable of efficiently catalyzing alkaline HERs, using a sequential catalytic polymerization-ionic reduction-thermal pyrolysis strategy.The positive modulation of Fe sites on the catalytic interfacial and surface properties of alkaline Ru nanoparticles has been investigated by electrochemistry, in-situ ATRIR, in-situ Raman, density-functional-theory (DFT) calculations, and ab initio molecular dynamics (AIMD) simulations, the positive modulation of Fe sites on the catalytic interfacial and surface properties of alkaline Ru nanoparticles was investigated. The results showed that Fe sites could regulate the distribution of interfacial water molecules and weaken the hydrogen bonding network on the catalyst surface, thus promoting the adsorption and activation of interfacial water molecules. Meanwhile, the H adsorption kinetics of the electron-rich Ru sites were optimized to a near-ideal state, forming an acid-like microenvironment rich in hydrated hydrogen ions. In addition, the interfacial hydroxides had a high affinity for binding to Fe sites, which alleviated the site blockage caused by hydroxide adsorption at Ru active sites. With the adsorption of OH on the Fe sites, the energy barrier for hydrolysis dissociation on the Ru sites decreased significantly.
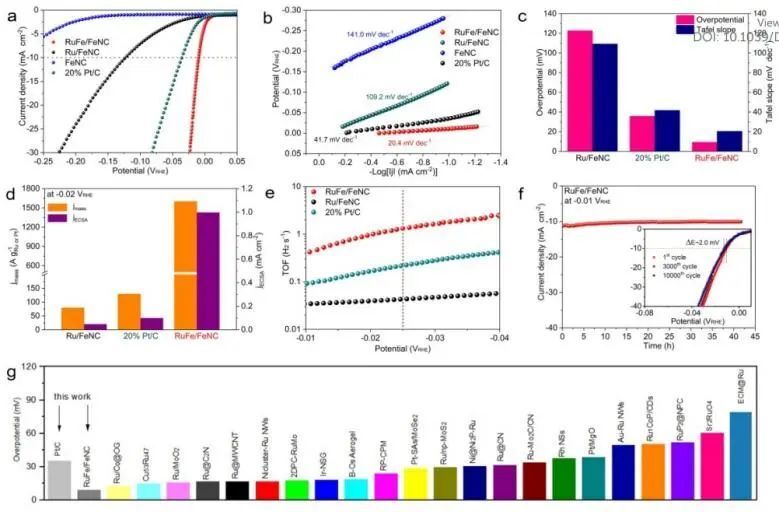
The results of the performance tests showed that under alkaline conditions, the RuFe/FeNC catalyst required a HER overpotential of only 9.3 mV to reach a current density of 10 mA cm-2, and a turnover rate (TOF) of 1.35 H2 s-1 at -0.025 VRHE, which was better than that of 20% Pt/C (35.7 mV, 0.21 H2 s-1). Furthermore, in addition, RuFe/FeNC was continuously stabilized by electrolysis at a potential of -0.01 VRHE for more than 40 h. The morphology and structure of the material remained essentially unchanged after the stability test, showing excellent stability. Overall, this work overcomes the limitations of slow hydrolysis dissociation, low proton supply and poor adsorption/desorption of key intermediates in alkaline HER processes, and also provides a new perspective to improve the HER performance by controlling the interfacial structure through doping.
