1.Background
Polymetallic oxides (polyacids) are a class of transition metal oxides with rich structural and physicochemical properties. Among them, Anderson-type polyacids are highly regarded for their excellent self-adaptation. By means of covalent modification, organic molecules are skillfully introduced into the polyacid backbone, which not only brings new functions to the polyacids, but also makes full use of the synergistic effect between inorganic and organic components. In addition, this modification further expands the diversity of the polyacid framework and significantly enhances its assembly complexity in solution and solid-state environments. In recent years, supramolecular assemblies based on parent polyacids and organic macrocyclic molecules have attracted increasing attention, and their novel and diverse topologies have demonstrated potential applications in various fields such as catalysis and energy. As a first-generation organic macrocyclic molecule, supramolecular assembly between crown ethers and polyacids has been reported in several papers (Figure 1(a)). However, the supramolecular assembly of covalently modified polyacids with crown ethers has not been studied enough, and this area needs to be explored in depth.
2.Major Achievements
The supramolecular assembly of covalently modified Anderson-type polyacids with 18-crown-6-ethers was intensively investigated by the team of Prof. Yufei Song from Beijing University of Chemical Technology, China, which explored in detail the effects of covalent modification modification mode of Anderson-type polyacids as well as the species of antiequilibrium cations on the supramolecular assembly. Through careful design, they successfully synthesized seven structurally unique polyacid-crown-6-ether complexes, and the structures of these compounds were exhaustively characterized using a variety of techniques such as single-crystal X-ray diffraction, ESI-TOF-MS, and 1H NMR. It is revealed that the 18-crown-6-ether complexed with alkali metal ions can act as a super cation and have complex interactions with covalently modified Anderson-type polyacids, thus forming supramolecular assemblies with different structures and compositions based on different covalent modifications. In particular, five of these supramolecular assemblies exhibit novel inorganic-organic rotamer structures. This study not only provides new ideas for the construction of polyacid-based supramolecular assemblies, but also lays a solid foundation for exploring their potential functional applications.
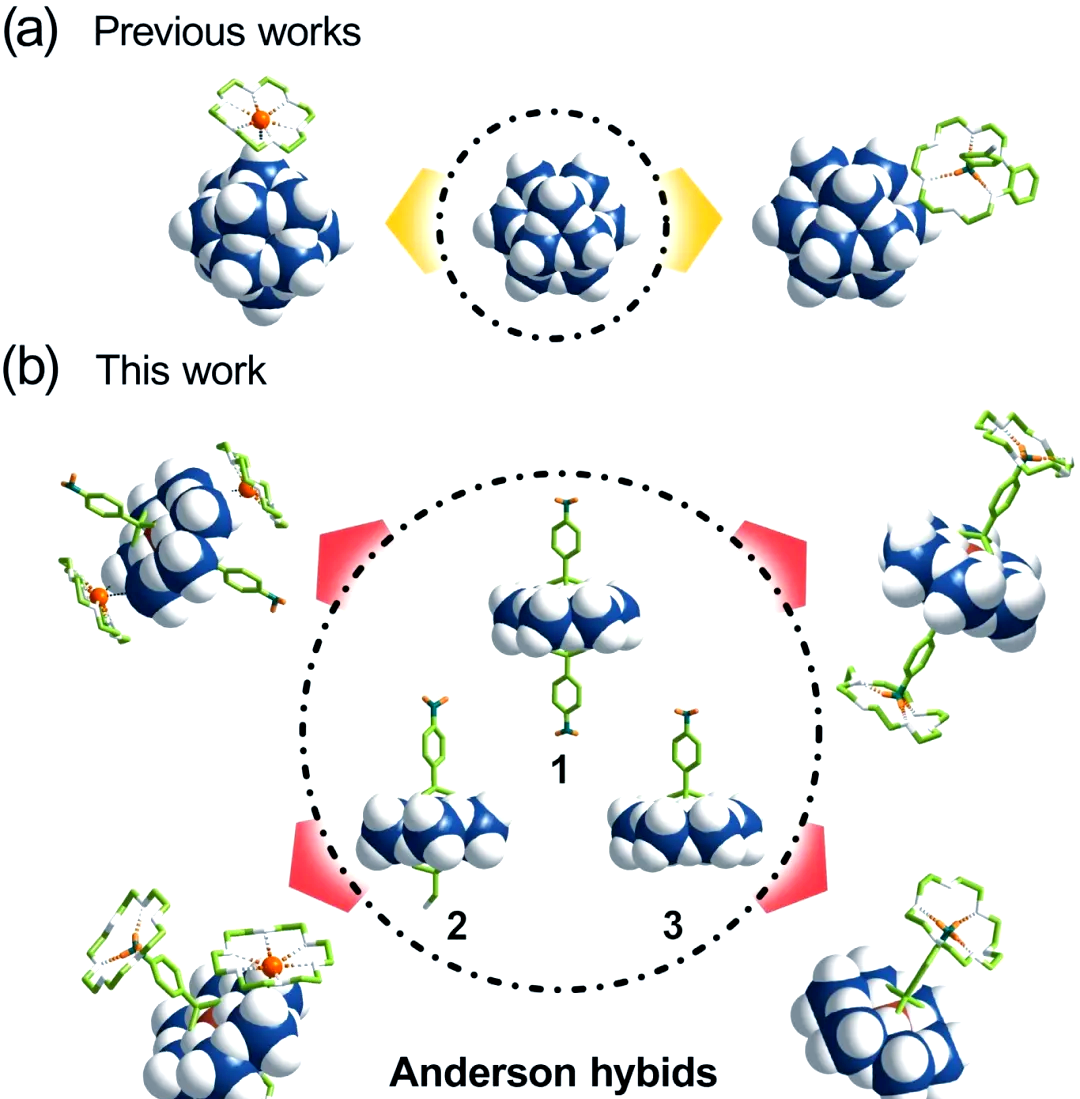
Figure 1. (a) Structural diagram of the known supramolecular assemblies of Keggin-type parent polyacids with crown ethers, (b) Schematic diagram of the supramolecular assemblies of covalently modified Anderson-type polyacids with crown ethers explored in this study.
3.Graphic guide
In this study, we successfully synthesized three covalently modified Anderson-type polyacids, which are compound 1 with bilateral symmetry, compound 2 with bilateral asymmetry, and compound 3 with unilateral asymmetry (see Fig. 1(b)). By means of an ion exchange technique, we replaced the antiequilibrium cations of these Anderson-type polyacids from tetrabutylammonium bromide (TBA) to alkali metal ions, such as sodium and potassium, which significantly increased the solubility of these compounds in water.
In the crystal structure diagram, we can observe that the super cation [(H2O)2(Na)(18-C-6)]+ shows a regular distribution in the two-dimensional network formed by compound 1 and the coordinating sodium ion. This ordered arrangement not only ensures charge balance, but also facilitates the tight stacking of single cells and the solid formation of crystals.
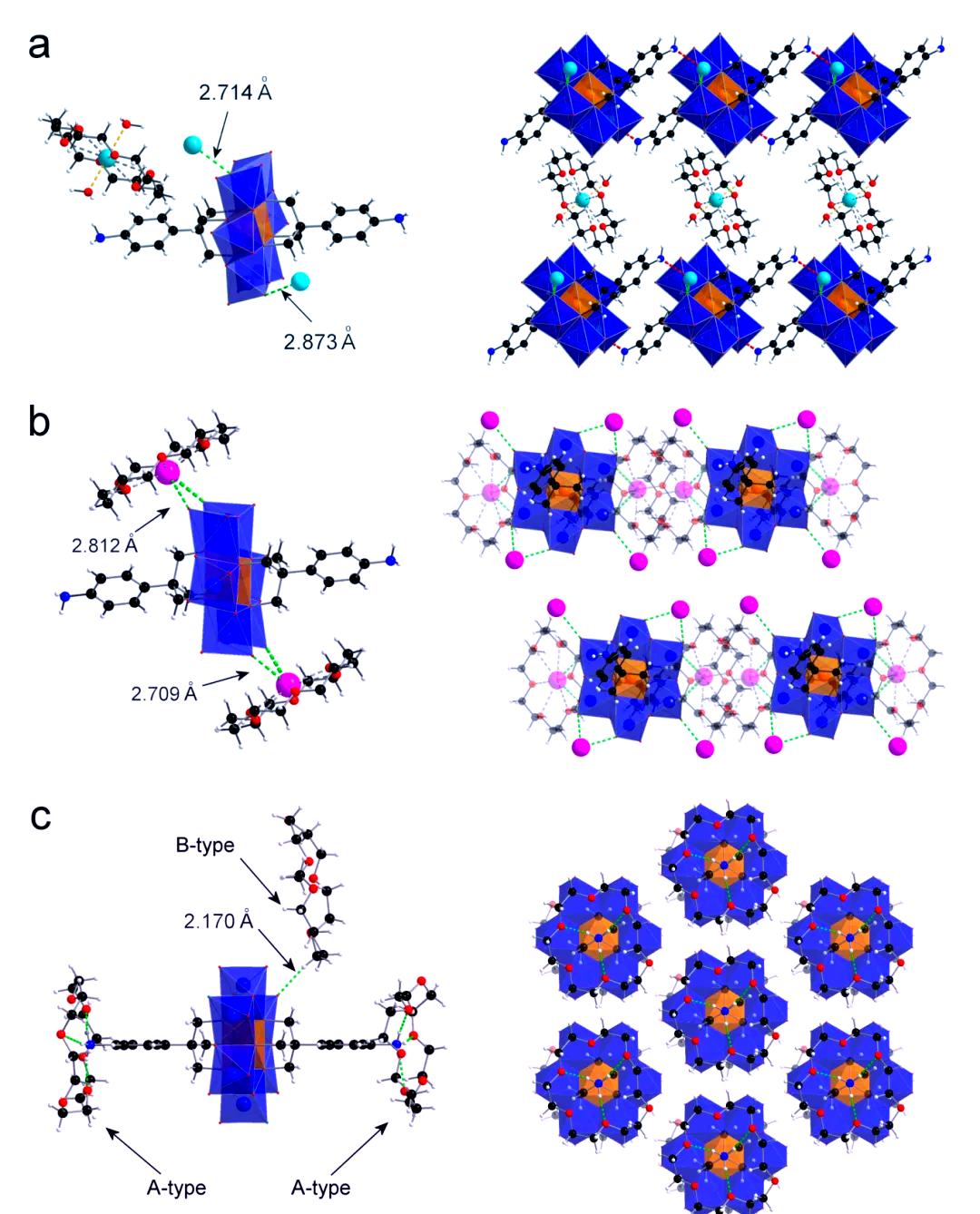
Single-crystal X-ray diffraction analysis revealed the presence of two different types of 18-crown-6-ether molecules in this type of inorganic-organic rotaxane structures (Fig. 2(c)). Among them, the A-type crown ether is interconnected with the protonated compound 1 through N-H∙∙∙∙O hydrogen bonding, thus building a sandwich-type quasi[3] rotane structure. The B-type crown ether, on the other hand, may have captured a protonated water molecule and interacted with the end-group oxygen of the Anderson-type polyacid in a strong C-H∙∙∙O hydrogen bonding interaction. These supramolecular complexes exhibit a hexagonal ordered stacking pattern in the c-axis direction.
In order to deeply investigate the effect of the covalent modification mode of Anderson-type polyacids on supramolecular assembly, the research team further explored the assembly behaviors of asymmetrically modified compounds 2 and 3 with 18-crown-6-ether. Taking the bilaterally asymmetrically modified compound 2 as an example, they found that the protonated aniline moiety encapsulated by 18-crown-6-ether was present in the supramolecular assemblies formed in the absence of additional protonating reagents, irrespective of whether the antimetabolite cation was sodium or potassium (see Figures 3(a) and 3(b)). This finding further confirms that the antiequilibrium cation has a significant effect on the interaction between inorganic and organic components under the same experimental conditions. To investigate the supramolecular assembly of compound 2 with 18-crown-6-ether in solution in more depth, the researchers also performed 1H-NMR and ESI-TOF-MS experiments.The results of ESI-TOF-MS showed that the asymmetric compound 2, compared to the symmetric compound 1, was more prone to protonation and deprotonation in aqueous solution. protonation in aqueous solution, a conclusion strongly supported by the ion and fragmentation peaks of the corresponding molecules.
Finally, the team further explored the supramolecular assembly behavior between the unilaterally asymmetrically modified compound 3 and 18-crown-6-ether.
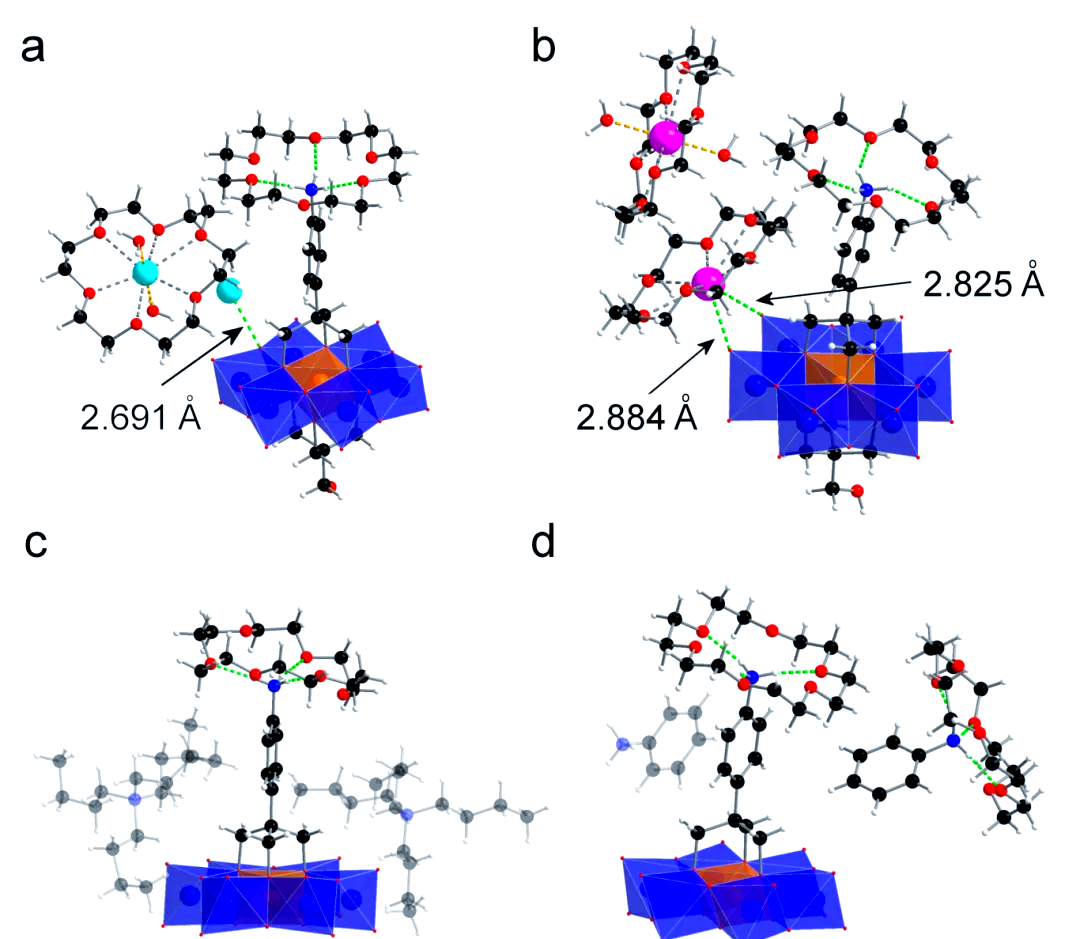
In summary, the supramolecular assembly of Anderson-type polyacids with crown ethers based on covalent modifications is explored for the first time in this paper. It is shown that the covalent modification mode of the Anderson-type polyacids and the antiequilibrium cations significantly affect the supramolecular assembly. Specifically, the asymmetrically modified polyacids are easily protonated upon interaction with 18-crown-6-ether, resulting in the formation of polyacid-crown-ether quasi[2]rolane structures in a 1:1 ratio, while the symmetrically modified polyacids need to be externally reinforced with an external reinforcement acid to generate sandwich-type quasi[3]rolanes. In addition, the coordination environments between the counterbalancing cations (e.g., sodium and potassium) of the polyacids and the polyacid anions also vary depending on the mode of covalent modification, which in turn affects supramolecular assembly. These studies provide new ideas for the assembly and functional application of covalently modified polyacids with organic macrocycles.
4. Author's profile
Changhan Lin is currently an associate professor at the School of Chemistry, Beijing University of Chemical Technology, and also serves as a tutor for master's students. He received his B.S. degree from Beijing University of Chemical Technology in 2010 and his Ph.D. degree under the supervision of Prof. Yufei Song in 2015. On his academic path, Changhan Lin was awarded a CSC scholarship in 2019 and has joined hands with Prof. Leroy Cronin from the University of Glasgow to explore the field of 3D printing and automated synthesis. His research interests are wide-ranging and cover a number of cutting-edge areas such as supramolecular self-assembly, polyacid-based organic-inorganic hybridization, and photoresponsive materials. Up to now, Changguan Lin has successfully undertaken one national natural science youth project, one central university fund, and published more than 30 academic papers as the first author or co-correspondent, as well as edited three monographs, which are fruitful.
Dr. Yufei Song, Doctor of Science, is a professor and doctoral supervisor, as well as the recipient of the National Outstanding Youth Fund and the director of the State Key Laboratory. His research interests include polyacid molecular assembly and functional materials, intercalation assembly and environmental catalysis, as well as the design and preparation of inorganic functional materials for energy and environmental protection. Prof. Song has published more than 340 SCI academic papers in renowned journals such as Nature Protocol., Nature Commun., Angew. Chem. Int. Ed. and J. Am. Chem. Soc. and has successfully granted more than 40 Chinese invention patents. In addition, he has edited one English monograph and co-edited three Chinese and English monographs. In his research projects, Prof. Song has assisted in the 973 Program projects, and chaired a number of projects commissioned by the state and enterprises, such as the Joint Fund Integration Project of the National Natural Science Foundation of China (NSFC) and the Distinguished Youth Project. His academic achievements have been recognized by the China Petroleum and Chemical Industry Federation (CPCIF) with a number of awards, including the “Outstanding Contribution to Youth Science and Technology Award”, and was selected as one of the “Young and Middle-aged Science and Technology Innovation Leaders” by the Ministry of Science and Technology (MOST). Currently, he serves on the editorial boards of Science Bulletin, Polyoxometalates and Catalyst.
Yi-An Yin, Wu-Ji Chen, Chun-Yan Liu, Meng-Meng Zhang, Chang-Gen Lin*, Haralampos N. Miras, and Yu-Fei Song*. Probing the covalently modified supramolecular assembly of Anderson-type polyacidic metal complexes with crown ethers to construct pseudo-rotational axis structures. Polyacid Metal Complexes, 2025, 4(1): 9140081.
