About the Author
Prof. Xu Jiaxi
Beijing University of Chemical Technology
Main research areas:
Organic Chemistry and Chemical Biology: (1) asymmetric catalytic synthesis, including the design and synthesis of novel chiral ligands and chiral catalysts, and the development of new asymmetric catalytic reactions; (2) synthesis of chiral drugs and their intermediates, and synthesis of chiral natural and non-natural compounds; (3) stereochemical studies on the synthesis of beta-lactam analogues; and (4) biologically active amino acids and peptides synthesis.
Article Introduction
Salen ligands are an important class of ligands that have been widely used in asymmetric catalytic organic reactions. Recently, Prof. Jiaxi Xu's group from Beijing University of Chemical Technology synthesized a novel enlarged Salen ligand containing flexible chains using L-phenylalanine, ethane/propylenediamine and salicylaldehyde, and successfully used it in the scandium-catalyzed enantioselective Michael addition reaction of indole and enone (2-cinnamoylpyridine 1-oxide). The catalytic system exhibits excellent reactivity and stereoselective control of the electron-rich indole substrate with yields up to 99% and enantiomeric excess percentages up to 99%. Meanwhile, the coordination ability of this new Salen ligand can be tuned by the electronic effect of substituents on the salicylaldehyde molecule, thus contributing to the construction of an excellent chiral environment in the scandium(III)-catalyzed asymmetric Michael addition reaction of indole with 2-cinnamoylpyridine 1-oxide.
Research
Synthesis of Salen ligand L
In the ring-expanding Salen ligands synthesized as shown in Figure 1, the original cyclohexane linkage chains were replaced by linear linkages consisting of rigid bisamides attached to flexible ethylidene-1,2- or propylidene-1,3- groups, which enlarged their coordination cavities to facilitate coordination with rare earth metal cations. The efficiency of the synthesis method and the yield of the required ligands L0-L7 are high.
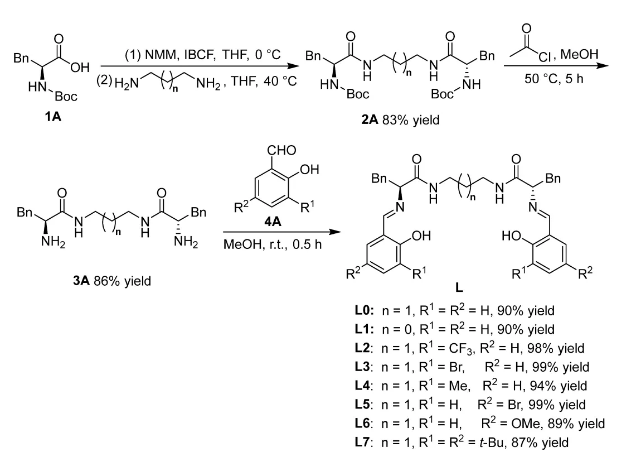
Figure 1. Synthesis of ring-expanding Salen ligand L
Influence of the structure of the ring-expanding Salen ligands on the enantioselectivity in the 1,4-conjugate addition reactions of indoles and unsubstituted α,β-unsaturated ketones
In order to investigate the effect of the structure of ring-expanding Salen ligands with flexible and rigid binding linkage chains on the enantioselectivity in 1,4-conjugate addition reactions of indoles and unsubstituted α,β-unsaturated ketones, the reaction of 6-methoxyindole (1a) and 2-cinnamoylpyridinium 1-oxide (2a) was chosen as a model reaction for evaluation of ligands (Table 1) and optimization of reaction conditions (Table 2).
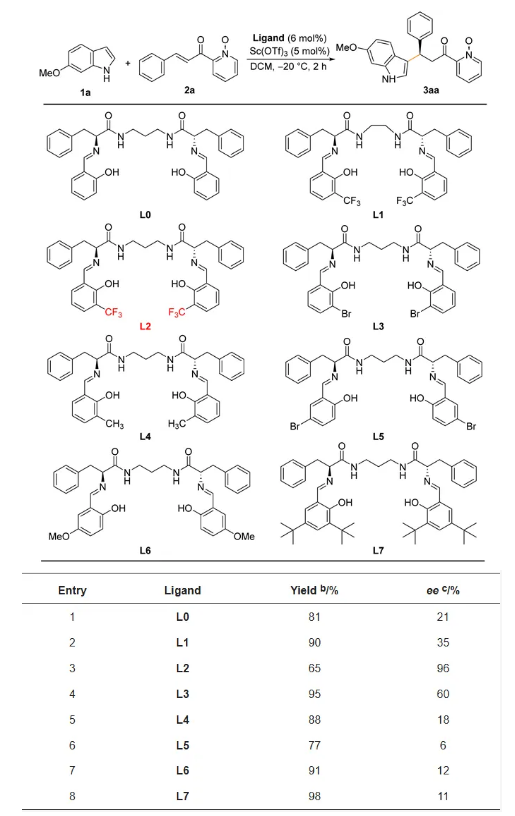
Table 1. Screening of ligands for asymmetric Michael addition of indoles and enones
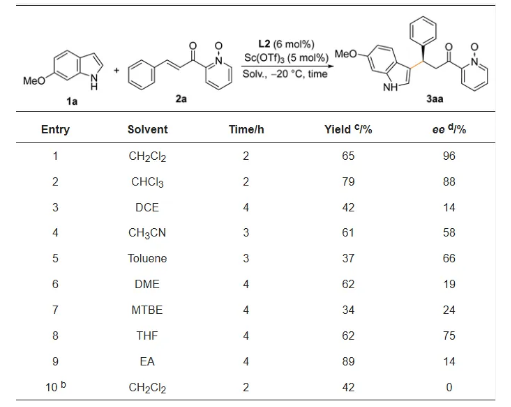
Table 2. Optimization of reaction conditions for asymmetric Michael addition of indoles and enones
Amplification of Salen ligands and complexes of Sc(OTf)3
Protected with nitrogen, the ligand L3 and Sc(OTf)3 were placed in anhydrous tetrahydrofuran and stirred at room temperature for 2 hours. After removal of excess solvent under vacuum, the filtrate was washed with dry dichloromethane and then recrystallized from the tetrahydrofuran/dichloromethane solvent mixture to give the complex L3-Sc(III). The structure is shown in Figure 2, which provides indirect evidence for a catalyst in the asymmetric Michael addition reaction.
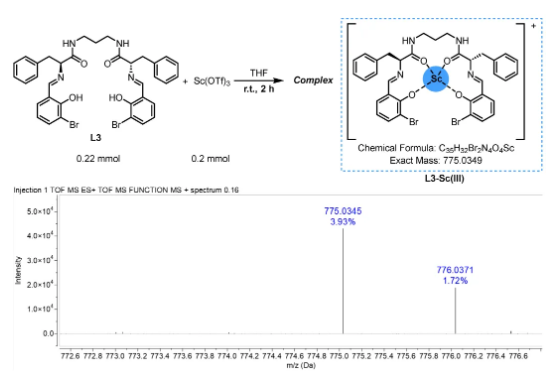
Fig. 2. Preparation of L3-Sc(III) complex and its HRMS spectrum analysis
Conclusion
In this study, a series of enantiomers containing flexible and rigid binding linkage chains of the expanded ring Salen ligand, which is more suitable for coordination with rare earth elements with larger radii, were prepared. At the same time, the electronic effect on the ligand coordination site was modulated by salicylaldehyde partial substituents. The results show that the ring-expanding Salen ligand linked to a flexible and rigid binding linker chain containing a propylidene-linked diamide and a strong electron-absorbing trifluoromethyl substituent is capable of achieving high activity and excellent enantioselectivity in the catalytic asymmetric Michael addition reaction of indole and 2-cinnamoylpyridine 1-oxide, which provides an effective method for future optically active modification of the 3-position of indole.
