
Co-first authors: Jennifer Zhang, Beijing University of Chemical Technology; Guanyi Zhang, Beijing University of Chemical Technology
Correspondence should be addressed to Yusen Yang, Beijing University of Chemical Technology, and Min Wei, Beijing University of Chemical Technology.
Correspondence should be addressed to State Key Laboratory of Effective Utilization of Chemical Resources, Beijing University of Chemical Technology, China.
DOI: 10.1021/acscatal.4c07190
A Pt1Con single-atom alloy (SAA) catalyst was prepared by Prof. Min Wei's group at Beijing University of Chemical Technology (BUCC) using the strategy of structural topological transformation of hydrotalcite (LDH). The role of this catalyst in the preparation of high value-added cyclopentanol (CPL) reaction by aqueous phase hydrogenation rearrangement of biomass platform molecule furfural (FAL) was investigated. A joint study based on reaction kinetics, isotope labeling tracer experiments, EPR and in situ FT-IR confirmed the five-step sequential tandem reaction pathway for the generation of CPL. This study provides new ideas for the preparation of high value-added fine chemicals by biomass green catalysis.
Background
Upgrading renewable biomass and its derivatives into fuels and fine chemicals through environmentally friendly catalytic processes is an effective strategy to alleviate the pressure on fossil fuels. Furfural (FAL) is a key biomass-derived platform molecule for the production of various high value-added chemicals. Among the downstream products derived from FAL, cyclopentanol (CPL) has attracted great interest due to its wide range of applications in the perfume, pharmaceutical, and biofuel industries.Aqueous-phase hydrogenation rearrangement of FAL provides a clean, efficient, and sustainable alternative for the production of CPL from renewable resources. In addition, the use of water as a solvent offers great advantages in terms of environmental protection and economic viability compared to organic solvents. However, the complexity of the tandem hydro-rearrangement route poses significant challenges in achieving high selectivity and high yield of the target product. Therefore, rational design and preparation of novel catalysts are crucial for the development of aqueous-phase hydrorearrangement reactions.
Research starting point
In this paper, Pt1Con single atom alloy (SAA) catalysts were prepared using layered dimetallic hydroxides (LDHs) as precursors. The catalyst exhibited excellent catalytic performance for the preparation of cyclopentanol from furfural aqueous-phase hydrogenation rearrangement, a reaction (CPL yield >93% after considering carbon loss; TOF values up to 2257 h-1). The immobilization of Pt monoatoms on the surface of Co nanoparticles via Pt-Co coordination was confirmed using AC-HAADF-STEM, XPS and XAFS studies. Experimental studies (reaction kinetics, isotope labeling tracer experiments, EPR and in situ FT-IR) combined with theoretical calculations (DFT) showed that the furfural-to-cyclopentanol hydrogen rearrangement process undergoes a continuous five-step tandem reaction pathway, where water molecules are involved in the reorganization of the C-O bonds within the side chains. In addition, the Pt-Co interface effectively lowers the energy barrier of the cyclopentanone hydrogenation step in the furfural hydrogen rearrangement, thus accelerating this tandem reaction.
Graphical interpretation
CoAl-(PtCl62-)-LDHs precursors were prepared by co-precipitation, and after thermal reduction treatment in H2 atmosphere at 800 °C, CoAl-(PtCl62-)-LDHs were converted into PtCo bimetallic catalysts loaded on oxide carriers (Fig. 1a). In which Co nanoparticles (~3.6 nm) were uniformly distributed and anchored on the amorphous Al2O3 carrier. the EDS elemental maps of the PtCo bimetallic samples (Fig. 1b) as well as the corresponding AC-HAADF-STEM images (Fig. 1c, d) confirmed that the Pt atoms were dispersed on the Co nanoparticles at the atomic scale (Fig. 1d). The local and electronic structures of Pt1Con SAA were further investigated by XAFS characterization (Fig. 1e-i), which demonstrated the electron transfer from Co to Pt in Pt1Con SAA, further confirming that the atomically dispersed Pt constitutes a monatomic alloy in a Pt-Co coordination manner.
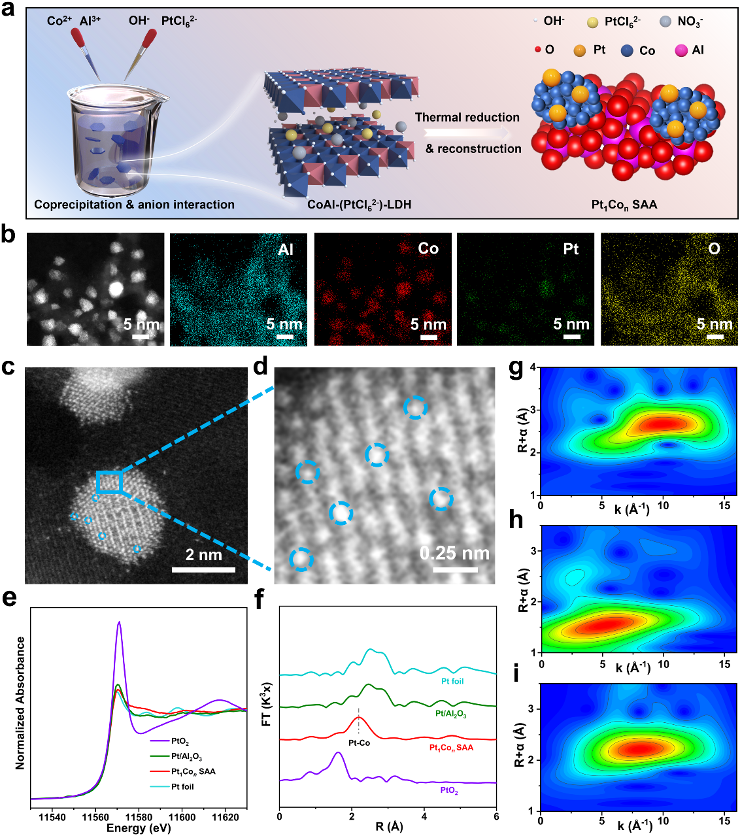
Figure 1: Electron microscopy study and fine structure characterization of Pt1Con SAA
As shown in Figure 2, the Pt1Con SAA catalyst achieved a high yield (93%; considering carbon loss) for the preparation of CPL by FAL aqueous-phase hydroreforming under optimized reaction conditions. No substantial decrease in catalytic activity or product yield was observed in five consecutive cycles, confirming the excellent aqueous phase stability of the catalyst. Based on the catalytic kinetic study, it was confirmed that the formation of CPL followed a consecutive tandem reaction route, i.e., the hydrogenation of the C=O bond in FAL to produce the intermediate FA, rearrangement of FA to produce 4-HCPE, dehydration and elimination of hydroxyl groups from 4-HCPE to form 2-CPE, hydrogenation of the C=C bond in 2-CPE to produce CPO, and hydrogenation of the ketone to produce the final product, CPL, in five steps ( Figure 2g). Water and cyclohexane solvent mixing experiments demonstrated that water molecules promote the reconstruction of C-O bonds within the side chains (Figure 2e). Subsequently, kinetic isotope tests of D2O substitution for H2O on Pt1Con SAA were designed under the same reaction conditions (Figure 2f) to further investigate the role of water, however the weaker kinetic isotope effects confirmed that the breaking of the O-H bond in H2O and the subsequent hydrogen exchange were not the key steps determining the rate of CPL generation. Furthermore, the Pt1Con SAA catalyst is universal in the aqueous phase hydrogen rearrangement reaction of three bio-derived furan derivatives.
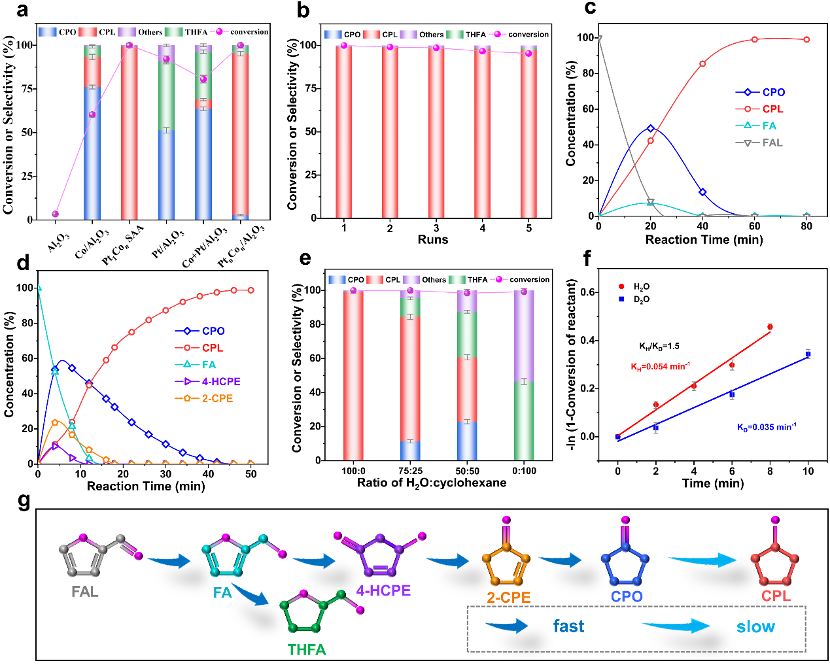
Figure 2: Performance evaluation of Pt1Con SAA catalyst for the preparation of CPL by aqueous-phase hydrorearrangement of FAL
The hydrorearrangement process of FAL and the key intermediate FA on the surface of the Pt1Con SAA catalyst was investigated by a combination of in-situ FT-IR experiments, NMR-MS spectroscopic studies, and DFT theoretical calculations (Figure 3). Evidence that the C=O bond in FAL undergoes activation adsorption and hydrogenation leads to the formation of FA was provided, validating the rationality of the reaction route proposed in Figure 2g. By using D2O as an isotope-labeled tracer in the reaction, the mechanism of hydrogenation of FAL was further investigated to promote the production of CPO/CPL via Piancatelli rearrangement with the participation of water molecules. The mass spectrometry results showed that H2O was indeed involved in the hydrogenation conversion of FAL to CPO; 1H NMR spectroscopy further confirmed the occurrence of hydrogen-deuterium exchange and keto-enol interconversion isomerism in the presence of the Pt1Con SAA catalyst, which verified that water was not directly involved in the hydrogenation reaction as a hydrogen donor.
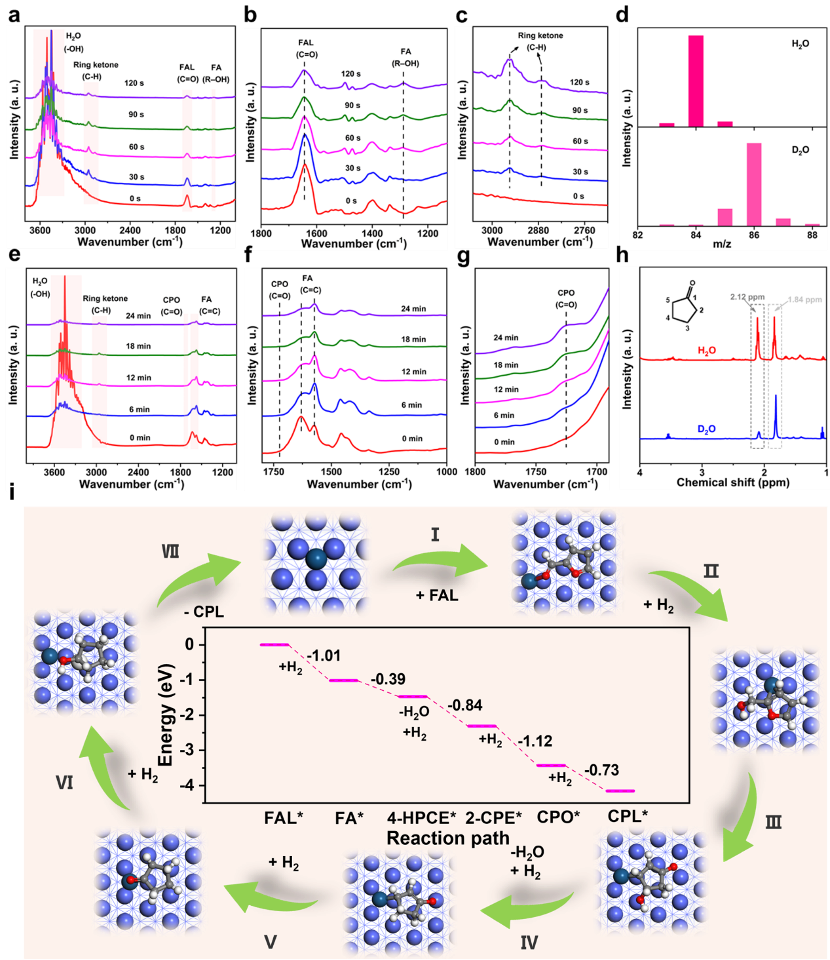
Figure 3: Mechanistic study of Pt1Con SAA for FAL aqueous-phase hydrogenation rearrangement reaction
Kinetic experiments were performed to determine the rate-determining step of the reaction (Figure 4), however, the values of the rate constants of the three reactions did not show significant differences, suggesting that there is no clear rate-determining step in this tandem reaction. Then, potential pathways for the hydrogenation of CPO on the surfaces of Co (111) and Pt1Con SAA (111) were calculated, and the results confirmed that the synergistic effect of the bimetallic sites in Pt1Con SAA effectively lowered the energy barrier of the CPO hydrogenation step, thus facilitating the conversion of CPO to the target product CPL.
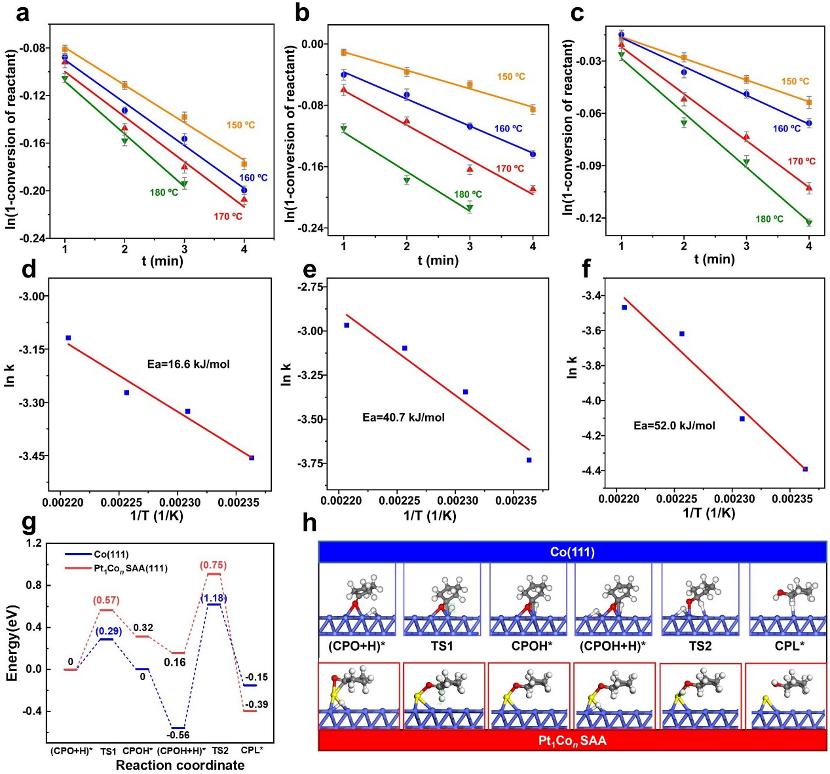
Figure 4: Kinetic study of hydrogenation rearrangement on Pt1Con SAA and calculation of the potential barrier for CPO hydrogenation
Summary and Prospects
In this work, Pt1Con single-atom alloy catalysts were prepared via an in situ topological transformation process, which exhibited excellent catalytic performance for aqueous-phase hydrogenation rearrangement of FAL (CPL yield >93%, considering carbon loss; TOF value: 2257 h-1). ac- Comprehensive HAADF-STEM, XPS and XAFS studies confirmed the immobilization of Pt monoatoms on the surface of Co nanoparticles via Pt-Co coordination. Experimental studies (reaction kinetics, isotope labeling tracking experiments, EPR and in situ FT-IR) combined with theoretical calculations (DFT) indicate that the hydrogen rearrangement process of FAL with CPL undergoes a consecutive five-step tandem reaction pathway, in which water molecules are involved in the reorganization of the C-O bonds within the side chains. In addition, the Pt-Co interface effectively lowers the energy barrier of the CPO hydrogenation step in the FAL hydrogen rearrangement, thus accelerating the tandem reaction. This work provides a useful exploration of the structural design and preparation of efficient catalysts for green aqueous phase reactions.
