Research Background
Carbonate-based electrolytes perform well in conventional lithium-ion batteries, thanks to their high ionic conductivity and wide electrochemical window. However, when such electrolytes are applied to new lithium-metal battery systems, they encounter a number of challenges, such as poor coulombic efficiency and the growth of lithium dendrites. Lithium nitrate (LiNO3) serves as an effective additive that can modulate the deposition pattern of lithium ions and contribute to the formation of a stable lithium-anode solid electrolyte interface (SEI) with a high lithium ion diffusion coefficient. Unfortunately, however, the solubility of LiNO3 in most carbonate electrolytes is extremely low (~10-5 g ml-1).
Recently, the team of Zhou Henghui from Peking University, Prof. Zhang Zhanjun from University of Chinese Academy of Sciences, Prof. Liu Wen from Beijing University of Chemical Technology, and Associate Prof. Zhou Mingyue from China University of Petroleum have explored a new method. They cleverly introduced a urea additive to promote the dissolution of LiNO3 in carbonate electrolytes by utilizing hydrogen bonding. This innovation significantly improves the performance of such electrolytes in lithium-metal batteries.
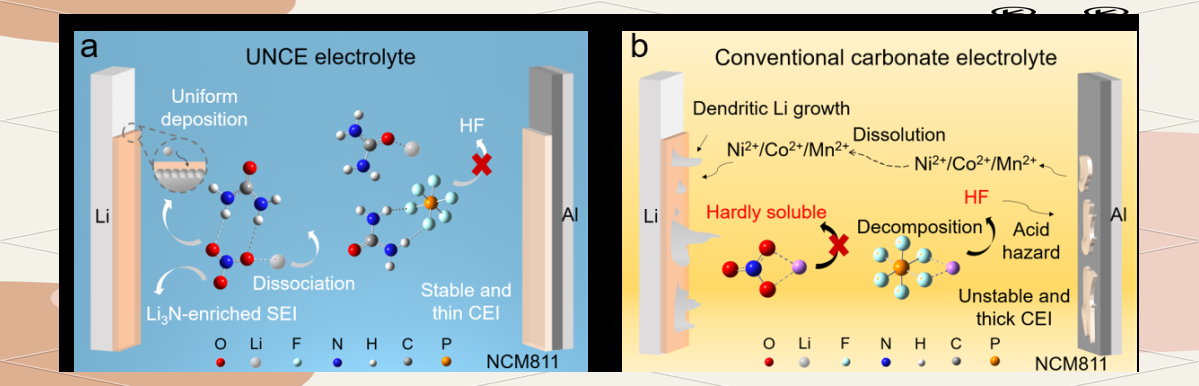
Fig. 1. Improvement of ester electrolyte by hydrogen bonding effect additive.
In this study, the dissolution of LiNO3 in the electrolyte was significantly promoted by the ingenious introduction of urea, which utilized the hydrogen bonding between its amino hydrogen and the oxygen of nitrate. This strategy not only contributes to the formation of stable nitrogen-rich SEI and effectively inhibits the growth of lithium dendrites, but also effectively inhibits the decomposition of LiPF6 in conventional carbonate ester electrolytes through the coordination of urea with lithium ions and the hydrogen bonding interactions with lithium salt hexafluorophosphate, which in turn reduces the generation of HF and protects the electrode materials from corrosion, thus comprehensively enhancing the performance of the battery.
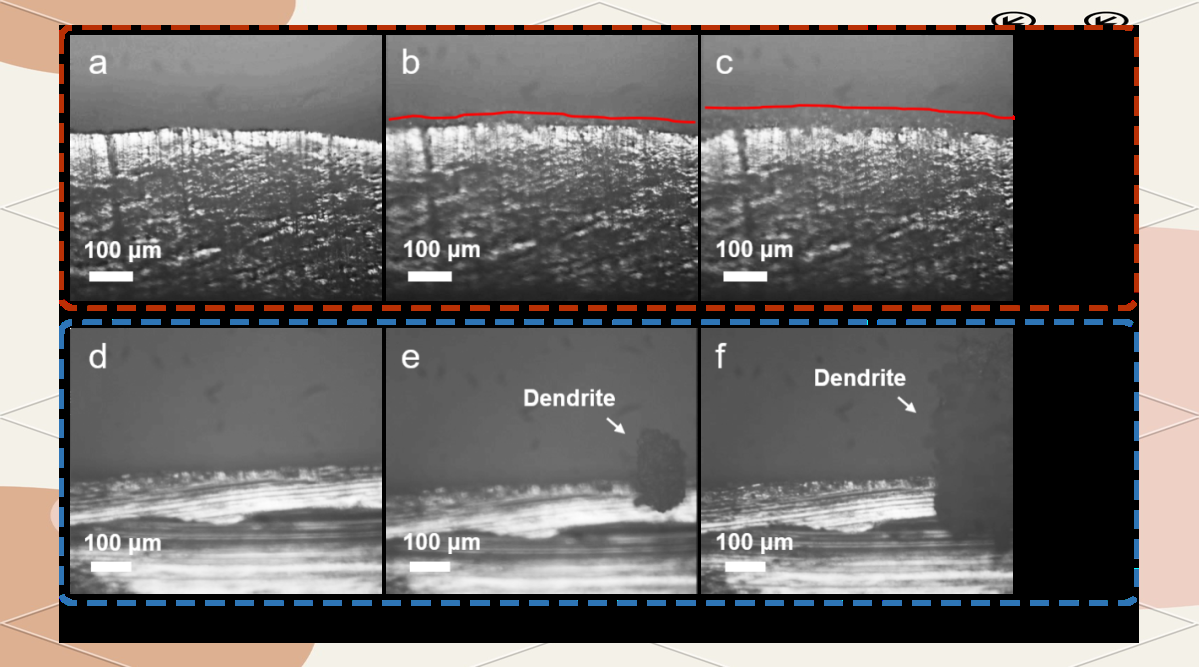
Figure 2. Optical microscopy reveals the influence of hydrogen bonding effect additives on lithium ion deposition behavior.
The study is titled "Tailoring solvation chemistry by hydrogen bonds in carbonate electrolytes for highly stable lithium-metal batteries (“Tailoring solvation chemistry by hydrogen bonds in carbonate electrolytes for highly stable lithium-metal batteries”) has been published in the Journal of Materials Chemistry A, a journal of the Royal Society of Chemistry, and has received wide attention and has been selected as a hot article.
Journal: J. Mater. Chem. A
Year: 20 Volume: Page: 15685-1 DOI: https://doi.org/1039/D4TA01535E
