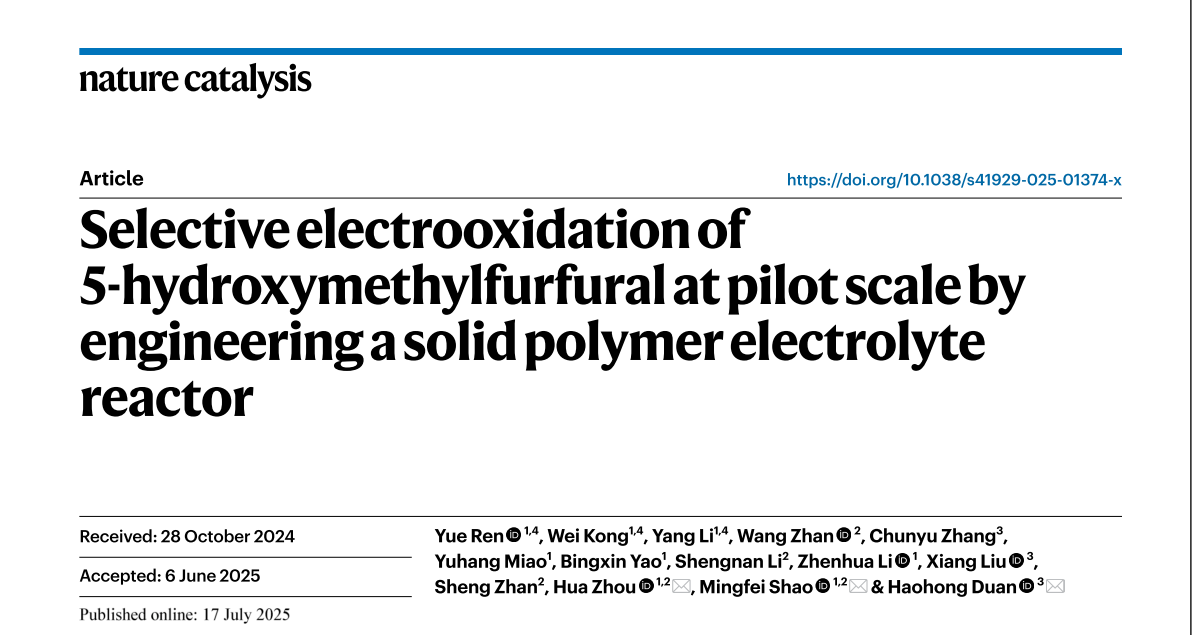
Here we show an engineered solid-polymer-electrolyte (SPE) reactor to steer Faradaic and non-Faradaic side reactions (Fig. 1), achieving FDCA production at industrially relevant current density (1.5 A cm−2), while maintaining high selectivity (97.0%), Faradaic efficiency (88.2%) and concentration (~1.24 M). The stability of the SPE-reactor was demonstrated in a continuous operation at 0.5 A cm−2 over 140 hours. Moreover, a 4.3 kW electrochemical platform was constructed with a scale-out strategy, reaching pilot-scale FDCA production rate of 33 kg per day. This work shows the capability of reactor engineering to enable selective and large-scale production of sustainable chemicals via electrochemical processes.
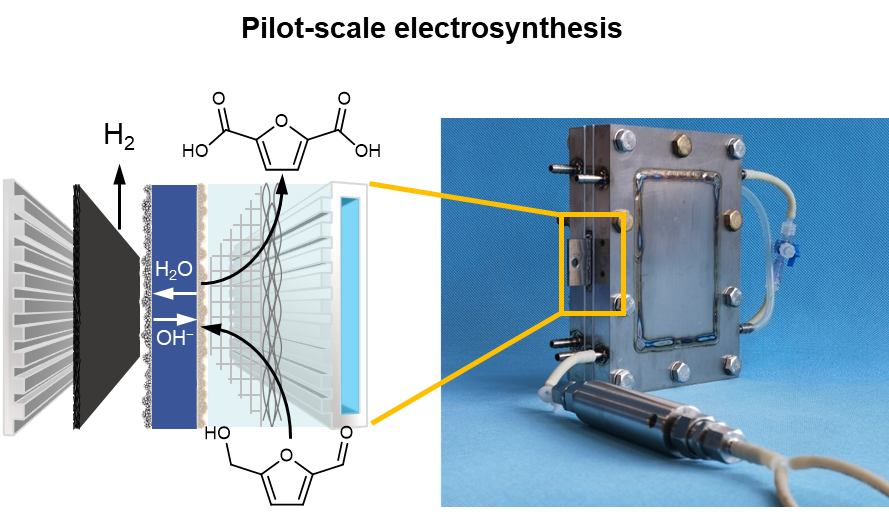
Fig. 1 Table of content
We conceived an SPE-reactor (and associated reaction systems) with systematic design. The amplified view of the SPE-reactor is depicted in Fig. 2a, which is consist of (from left to right): a cathode chamber (including cathode plate and GDL), a CCM, and an anode chamber (including PTL, anode plate with cooling chamber). Based on this design, a single-module SPE-reactor was assembled by positioning one cathode plate between two anode plates (in which electrolyte flows from front to back anode) that compacts the configuration and ensures a sampling function to probe reaction intermediates (Fig. 2b and c). Then, an electrochemical platform is established by integrating SPE-reactor with a static mixer and associated systems. Notably, there are four key components for the SPE-reactor and systems (Fig. 2d−g).
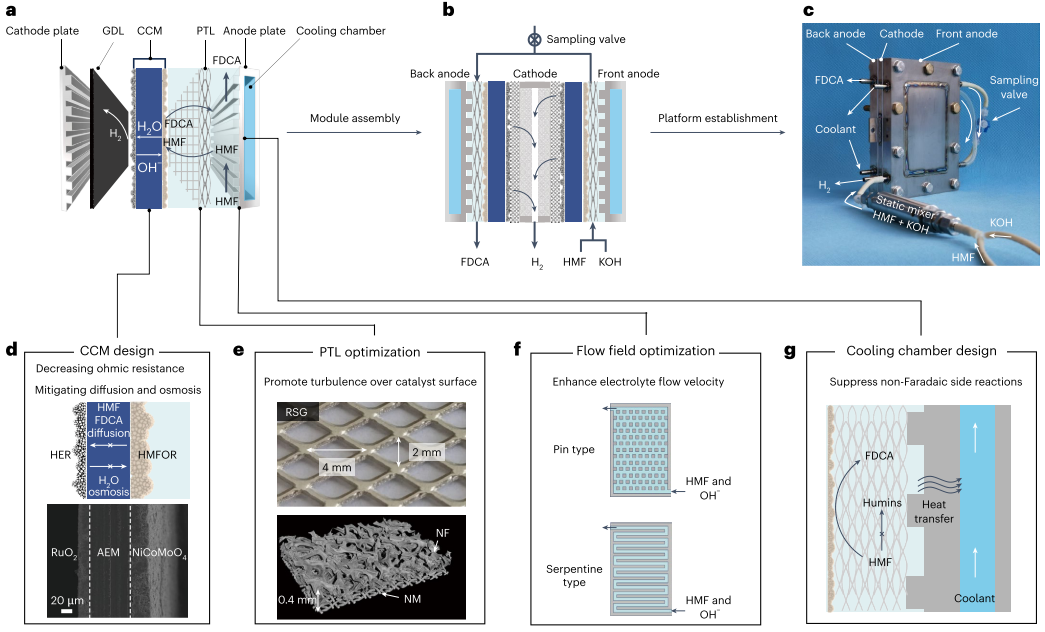
Fig. 2 Design and performance of solid-polymer-electrolyte (SPE) reactor. a, Schematic of the enlarged SPE-reactor configuration. It consists of a cathode for HER and an anode for HMFOR, an AEM-based CCM for water and OH– transport and catalyst support. b, Schematic of a single-module SPE-reactor. c, Photograph of single-module SPE-reactor connected to a static mixer. d, SEM image of the cross section of CCM. It consists of commercial RuO2 as HER catalyst and NiCoMoO4 as HMFOR catalyst, an AEM as the solid polymer electrolyte. e, Photographs of porous transport layer (PTL). It consists of a rhombic-shaped grid (RSG) layer (top) and a microstructural visualization of composited nickel foam (NF) and nickel mesh (NM) layer determined by Micro-CT (down). f, Illustration of pin- and serpentine-type flow field for anode.g, Illustration of cooling chamber integrated into anode plate.
Furthermore, we demonstrated an engineered solid-polymer-electrolyte (SPE) reactor and modular electrochemical platform to enable selective electrooxidation of high-concentration HMF to FDCA at kW-scale (4.3 kW, Fig. 3) and ampere-level current density (1.0 A cm−2). These results represent an important step toward large-scale electrosynthesis of sustainable plastic monomers from biomass. The key roles of the reactor include: suppressing Faradaic oxygen evolution by strengthening mass transfer; alleviating non-Faradaic HMF condensation; mitigating undesired crossover of molecules between cathode and anode.
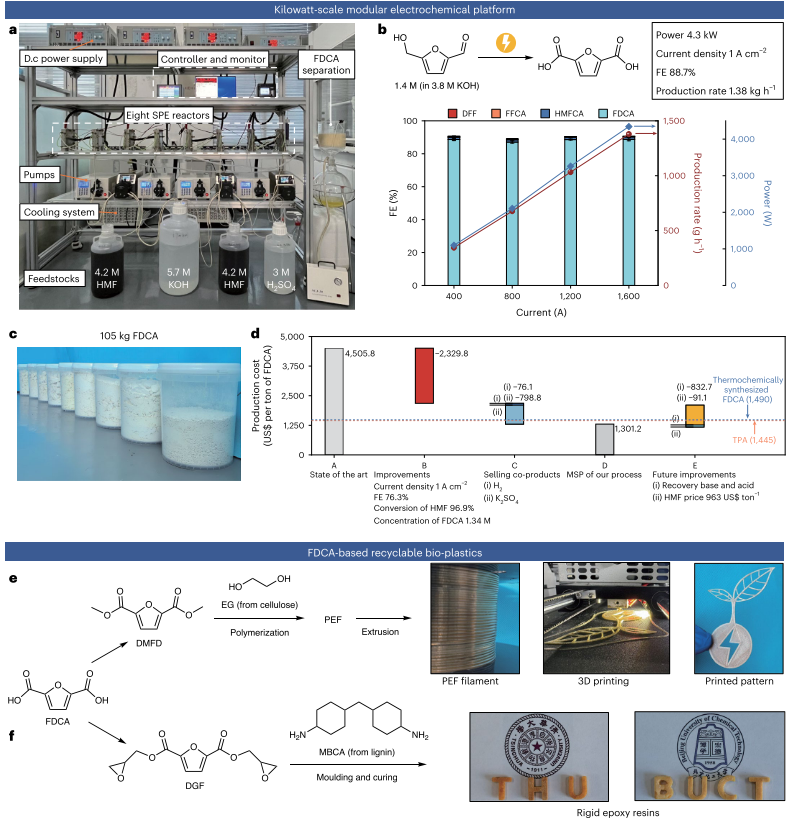
Fig. 3 | Kilowatt-scale FDCA production and its application in bio-plastics manufacturing. a, Photograph of the modular electrochemical platform. It consists of eight-SPE-reactors in parallel, associated feeding, cooling, powering, controlling and monitoring systems, and FDCA separation system.b, Catalytic performance (FEs of the products, FDCA production rate and power) at 1.0 A cm−2 in the platform containing different numbers of SPE-reactor (current of 400, 800, 1200 and 1600 A correspond to 2, 4, 6 and 8 modules, respectively). Error bars in this figure correspond to the standard deviation of three measurements. c, Photograph of the isolated FDCA in this work. d, Techno-economic assessment of HMF-to-FDCA cost based on the current reactor performance in this work and potential improvements. e, Manufacturing of FDCA-based PEF thermoplastic and its application in 3D-printing to generate customized patterns. f, Manufacturing of FDCA-based thermoset and its application in letter molding. The inset letters of THU is the abbreviation for ‘Tsinghua University’ and BUCT for ‘Beijing University of Chemical Technology’. The university badges are shown in the background for reference.
We propose that further research of HMFOR should aim at improving the scalability, stability, and economic feasibility. Continuous flow electrochemistry with larger reactors (larger working area, and more modules in serial or in parallel), system integration (feeding, electrolysis, product separation and associated units), and artificial intelligence-assisted automation (electrode fabrication, system monitoring and controlling, self-optimization) may enable acceleration of HMFOR industrialization. Advances in catalyst/electrode and ion exchange membrane will increase the stability as well as energy efficiency of the electrolysis process. Further optimization of electrolysis parameters will decrease the capital and operational costs of the process. Improvements in upstream HMF synthesis technology with low cost and using crude HMF as the feedstock could be a solution to lower material cost and thus the overall cost of electrochemically synthesized FDCA. Optimization of separation technology could also enable the isolation of polymer-grade FDCA for value-added polymers manufacturing, as well as for recovery of electrolyte for decreasing material cost.
Linkage: https://doi.org/10.1038/s41929-025-01374-x
