
Herein, we propose a “bubble seeding” strategy by introducing nanobubbles (NBs, ~200 nm in diameter) into electrolyte to promote the generation of macroscopic bubbles. The precursive nanobubbles can act as the beforehand nuclei with a certain supersaturation, lower the supersaturation barrier for further growth, and result in reduced overpotential as high as 130 mV for oxygen evolution reaction. The enhancement depends on nanobubble coverage and size, with higher coverage and larger sizes favoring macrobubble growth. The nanobubbles with inert gas species (e.g. N2 for oxygen evolution reaction) can also work as the seeds, while the interfering or consumable gas species (e.g. O2 for hydrogen evolution reaction) would hinder the generation of macroscopic bubbles and enlarge the overpotential. The water splitting device working at presence of nanobubbles exhibits stable operation voltage during repeated start-stop cycles in contrast to traditional electrolyte without NBs, indicating great potential of such traceless nanobubble additive strategy for stabilizing gas evolution applications.
By introducing NBs to the electrolyte, the OER onset potential of the Pt/Au electrode at the current density of 1 mA/cm2 is found to be 1.70 V (vs RHE), with a lowered overpotential (Δη) of 130 mV relative to the intrinsic onset potential (1.83 V) without bulk NBs. To further verify such promotion effect, a small amount of bulk NB solution (5 mL) was injected into the electrolyte (25 mL) at the equilibrium state (400 s) of OER at a rather limited potential of 1.7 V (vs RHE). As shown in Fig. 1d, there is an obvious current jump after the injection of bulk oxygen NBs solution, accompanied by the appearance of MBs on the electrode (Fig. 1d inset). The results suggests the introduction of bulk NBs can effectively lower the onset potential of OER, as a “traceless” additive to promote gas evolution reaction.
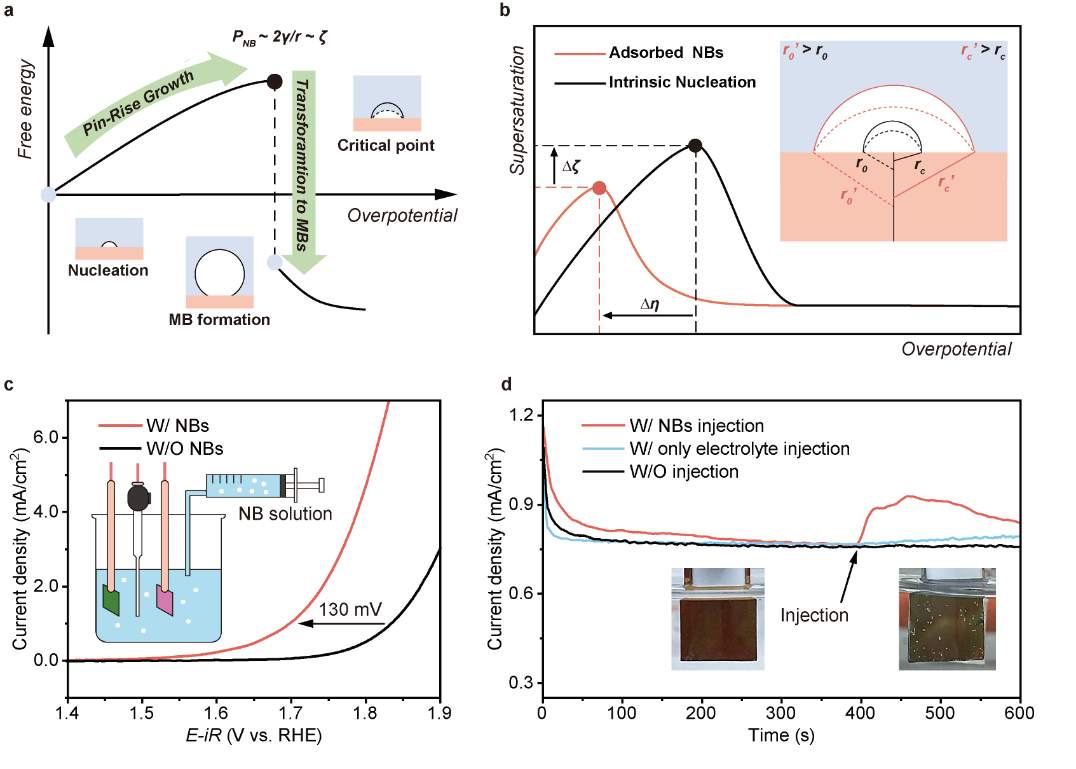
Fig. 1.NBs as traceless additive to promote gas evolution reactions. (a) Pin-rise growth of surface nanobubble during initial stage of gas evolution reaction. (b) Scheme of the relation between the supersaturation nearby the intrinsic generated (black), adsorbed (red) surface NBs and the overpotential during gas phase transformation. (c) OER polarization curves of the Pt/Au electrode (1×1 cm2) with (red) and without (black) the introducing of NBs (d) Current density variations at 1.7 V (vs RHE) after the injections of NB solution (red), electrolyte-only (blue), respectively, and without injection (black) (working area: 1.5×1.5 cm2).
By adjusting the adsorption time before OER tests, we found that the onset potential at 1 mA/cm2 shifts from 1.76 V to 1.70 V with increasing adsorption time (without adsorption of NBs, the onset potential is 1.83 V), verifying the promotion effect of introducing NBs (Fig. 2c). As taking a different perspective for such variation, the current density at the potential of 1.75 V (vs RHE) increases accordingly with the prolonging of NB adsorption time (Fig. 2d).
Besides, surface plasmon resonance microscope (SPRM) was used to observe the growth/detachment of processes at potential of -0.58 V vs RHE. As shown in Fig. 2e, a macrobubble approached its critical size for the detachment. After 50 ms (1.40 s), a gas nucleus with the size of ~10 μm remained. At this stage, even though the adsorbed NBs have transformed to macrobubbles, the gas nuclei at macroscale remained could still act as the generation site for the growth of next macrobubbles (7.65 s and 13.70 s). Since this is a buoyancy driven bubble departure process, the inevitably roughness/defects on the Pt/Au electrode would serve as the pining sites of the macrobubble. Upon departure, buoyancy and the adhesion force would induce a bubble neck which results in the incomplete departure of the macrobubble, leaving the gas nuclei. We elucidate that the adsorbed NBs can not only serve as the nuclei for the growth of MBs, but also help to form gas seeds for the successive growth of MBs after their detachment (Fig. 2e).
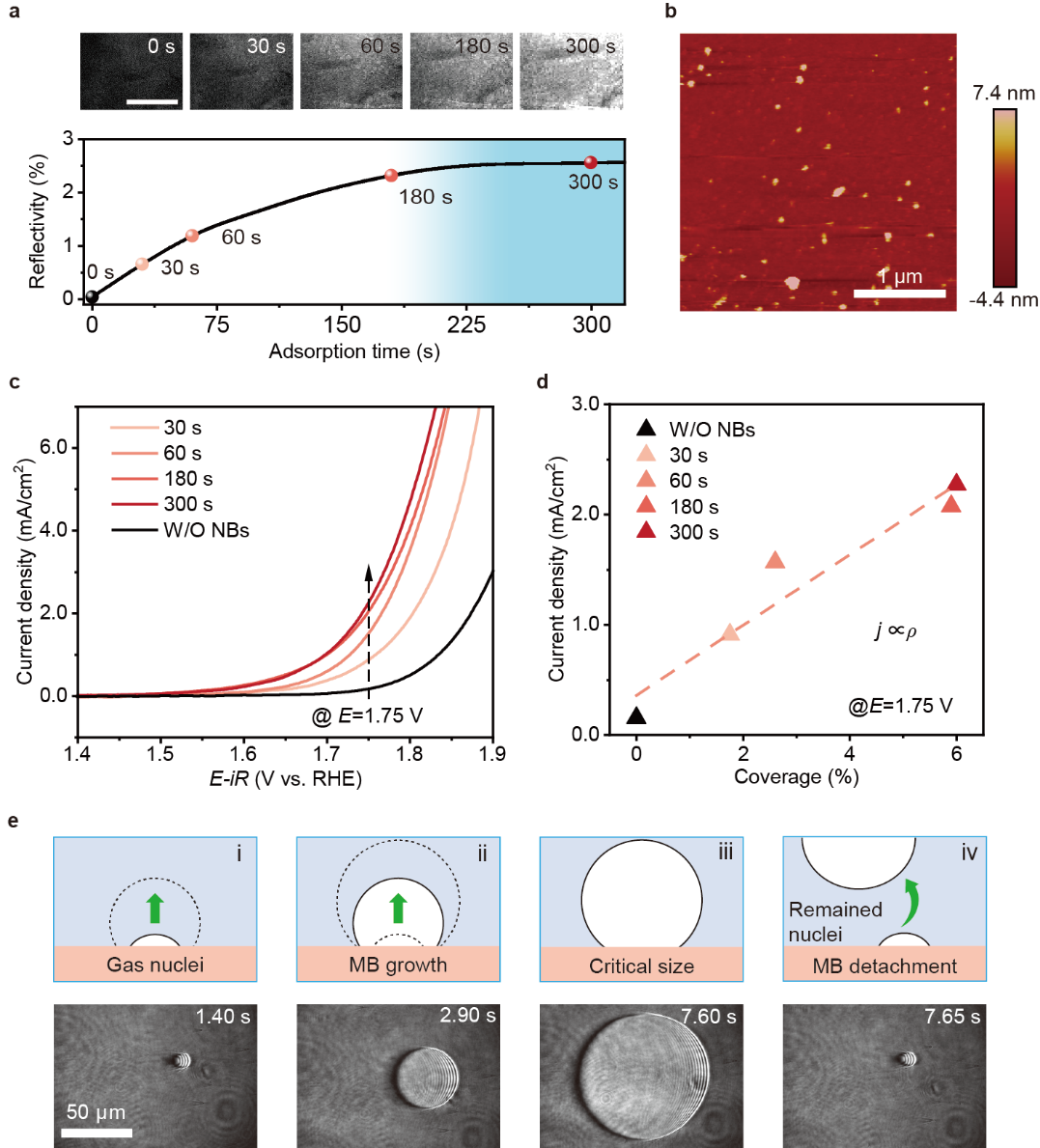
Fig. 2.Effect of nanobubble coverage on the onset potential of OER. (a) Time-dependent reflectivity variation with NBs’ adsorption, top: differential images of surface plasmon resonance at 0 s, 30 s, 60 s, 180 s and 300 s (Scale bar=400 μm). (b) Atomic force microscopy image of the electrode surface (3 × 3 μm2) with adsorption of NBs after 300 s. (c) OER Polarization curves of the Pt/Au electrodes (1×1 cm2) with different NBs’ adsorption time (colored) and without NBs’ adsorption (black).(d) Plots of current density (statistically derived from the Fig. 2D) and NB coverage at 1.75 V vs RHE. (e) In situ surface plasmon resonance microscope (SPRM) observation of macrobubble (MB) formation/detachment and the remaining gas nuclei.
Besides, the size influence is also critical for such promotion effect. Different sized bulk NBs were acquired by placing the fresh generated NB solution for different time. As shown in Fig. 3a, the average diameter of freshly generated NBs is ~235 nm and increases to ~290 nm within 10 h. Accordingly, the number density of NB solution decreases from ~11.0×107 particles/mL to 3.10×107 particles/mL. Such different sized NBs were diluted to maintain number density of ~3×107 and further introduced into the electrolyte for the adsorption. As shown in Fig. 3b, the corresponding polarization curves indicated that the OER onset potential decreases with the increase of introduced NB size. Like the trend of NB coverage, the current density also exhibits a positive correlation with r at the overpotential of 1.75 V. Also, the OER potential shifts from 1.76 V to 1.73 V at the current density of 0.4 mA/cm2 as the average r increases from 225 nm to 291 nm. Further calculation demonstrates that the OER overpotential decreases with decreasing adsorbed NB supersaturation ratio (Fig. 3c).
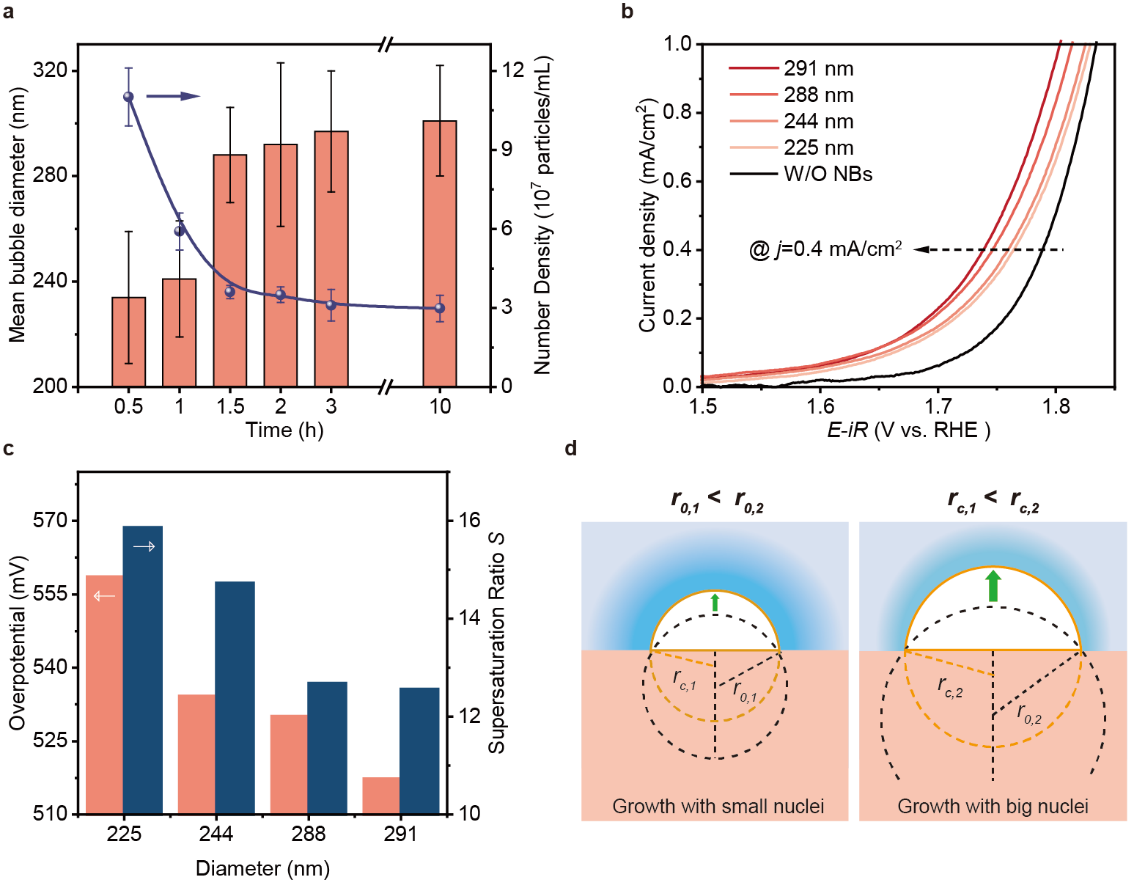
Fig. 3.Effect of nanobubble size on the onset potential of OER (a) Time-dependent variation of oxygen NB diameter and number density within 10 h. (b) OER polarization curves of the Pt/Au electrodes (1×1 cm2) with different size NBs’ adsorption (colored) and without NBs’ adsorption (black). (c) The OER overpotential at 0.4 mA/cm2 statistically derived from the Fig. 3B and corresponding calculated supersaturation ratio S of the adsorbed NBs with different sizes. (d) Schematic illustration of the difference in the transformation from NBs to MBs with small (left) and big (right) nuclei.
To further investigate the versatility of such NB additive strategy, other specific gas NBs were evaluated for OER and HER tests. Specifically, a series of gas species including N2, H2, O2 and air NBs were introduced into the electrolyte for OER. After the adsorption of NBs reached equilibrium (> 5 min), LSV tests were performed. As shown in Fig. 4a, it can be seen that the presence of nitrogen, oxygen and air NBs can lead to lowered onset potentials as compared with the solution without NBs (black line). Whereas H2 saturated solution and H2 NBs solution leads to enlarged onset potential for OER. Similar situation can be observed for HER (Fig. 4b), where solutions containing H2, N2 and even air NBs exhibit promotion effect, while the introduction of O2 saturated and O2 NBs solution cause the negative effect. It is reasonable that the introduced target or reaction-inert gas species (e.g. H2 or N2 for HER, O2 or N2 for OER) would promote the MBs’ formation, but the negative effect of reaction- interfering gas species (e.g. O2 for HER, H2 for OER) should be relative to the side reactions (ORR or HER).
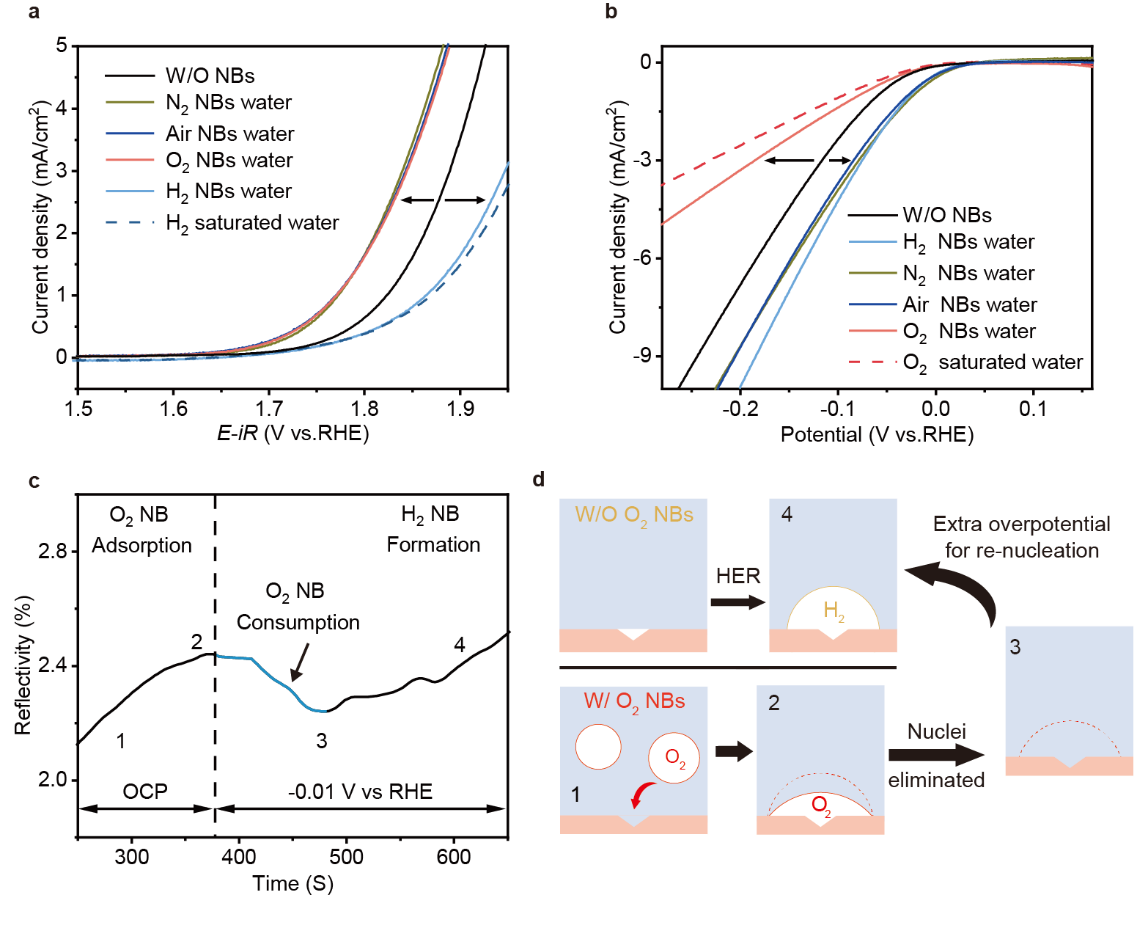
Fig. 4.Verification of gas species for the promotion of MBs. (a) OER Polarization curves of Pt/Au electrode (1×1 cm2) with different gas species. (b) HER Polarization curves of Pt/Au electrode (1×1 cm2) with different gas species. (c) Time dependent reflectivity variations of Pt/Au electrode with O2 NBs adsorption where OCP refers to open circle potential and the black dashed line denotes the start of given potential (-0.01 V vs RHE). (d) Schematic illustration of the plausible mechanism for the cathodic H2 seed removal at presence of O2.
To further verify the NB addition strategy for the adaption of the power fluctuations of renewable (wind and solar) energy in practical scenarios (Fig. 5a), we applied it in an anion exchange membrane (AEM) water splitting device. With Ar NBs as the additive, the constant current density was set as 200 mA/cm2 and the duration of both on and off was set as 100 s (Fig. 5b). The overall water splitting potential of the device remains stable at 1.54 V, but the scenario without introduction of NBs is 1.55 V initially (Fig. 5c and S18) and gradually increases to 1.59 V during 120 cycles of repeated start-stop operation.
Further LSV tests reveal that electrochemical activity can be well preserved for the electrodes after cycles at the presence of NBs. Besides, the performance degradation is also verified through the examination of double-layer capacitor (CDL) and Tafel slope measurements (Fig. 5g and 5h). For example, the CDL of the NiFe-LDH electrode, as depicted in Fig. 5g and S20, exhibits a decline from 4.40 mF/cm² to 3.26 mF/cm² after 300 cycles in the presence of NBs, which is in contrast to the sharper decrease to 2.34 mF/cm² for the absence of NBs. Similarly, the Tafel slope of the NiFe-LDH electrode after 300 cycles without NBs escalates from 58.86 mV/dec to 80.32 mV/dec. Conversely, for the electrode after cycles operated in the presence of NBs, the Tafel slope remains almost constant at 60.69 mV/dec (Fig. S21), indicative of sustained electrochemical activity.
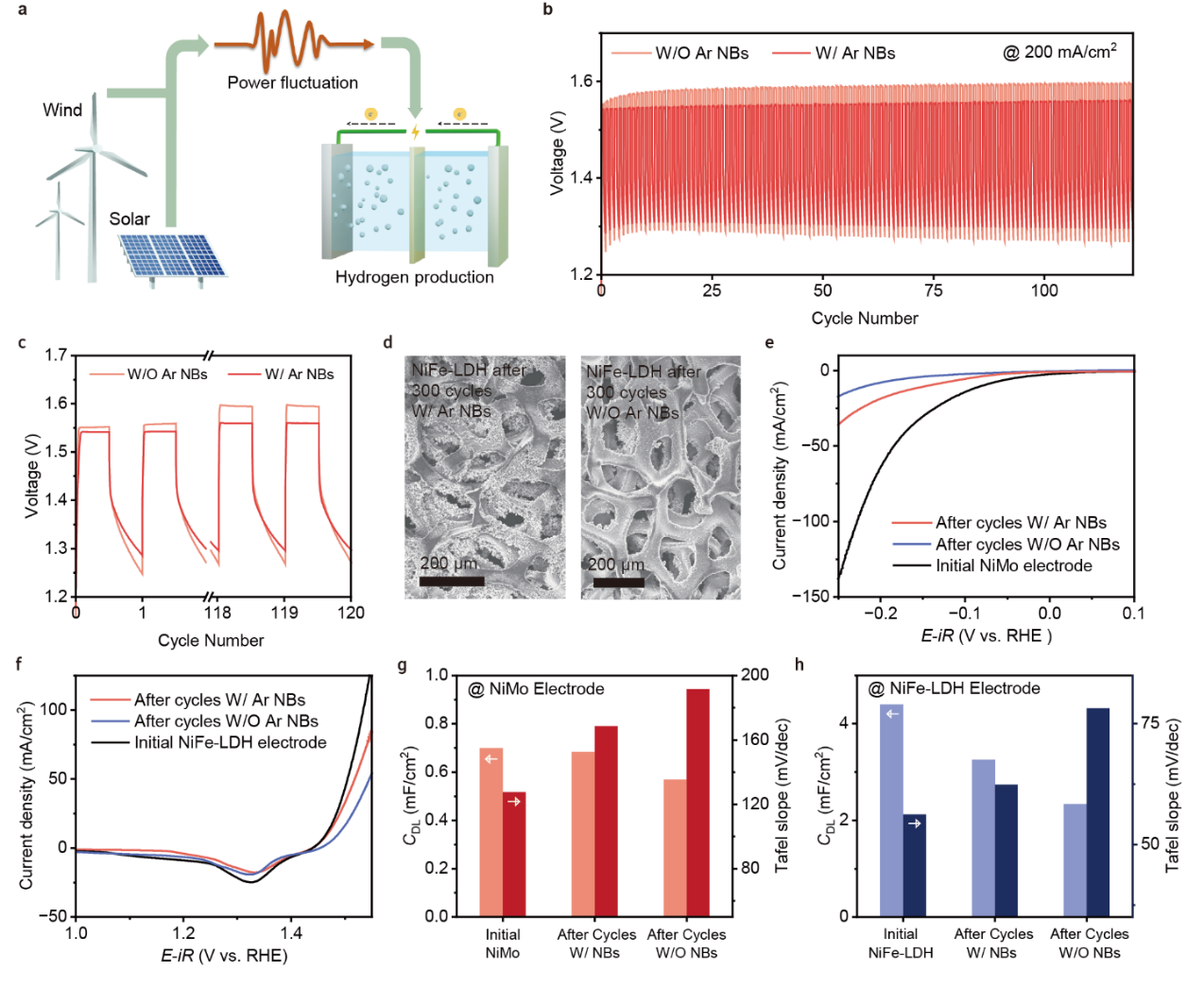
Fig. 5.Application for water splitting device with repeated start-stop operation. (a) Scheme of the power fluctuations of renewable (wind and solar) energy for hydrogen production devices. (b) Repeated start-stop cycles performance of AEM device with/without the introduction of Ar NBs to keep current density of 200 mA/cm2. (c) Voltage variation during repeated start-stop test with/without the introduction of Ar NBs. (d) SEM images of NiFe-LDH electrode after repeated start-stop cycles tests with and without NBs. (e) HER polarization curves of the as prepared NiMo electrode (black) and after 300 cycles of repeated start-stop operation with Ar NBs (red) and without NBs (blue). (f) OER polarization curves of the as prepared NiFe-LDH electrode (black) and after 300 cycles of repeated start-stop operation with Ar NBs (red) and without NBs (blue). (g) CDL and Tafel slope of the freshly prepared NiMo electrode (Initial NiMo) and NiMo electrode after repeated start-stop cycles operation with NBs (After Cycles W/ NBs) and without NBs (After Cycles W/O NBs). (h) CDL and Tafel slope of the freshly prepared NiFe-LDH electrode (Initial NiFe-LDH) and NiFe-LDH electrode after repeated start-stop cycles operation with NBs (After Cycles W/ NBs) and without NBs (After Cycles W/O NBs).
In conclusion, we propose a traceless “bubble seeding” strategy by introducing target (H2 and O2) or inert (N2) NBs into electrolyte to promote the generation of MBs. The adsorbed NBs on the electrode surface can work as the beforehand nuclei with a certain supersaturation, and lower the supersaturation barrier for MBs’ growth, resulting in reduced overpotential. With the introduction of NBs, the operation voltage of AEM device during repeated start-stop cycles can stable within 120 cycles, indicating great potential for water electrolysis applications using renewable fluctuated energy supply. Such traceless NB additive strategy paves an alternative way for devices themselves to adapt fluctuating renewable energy, and more importantly, promotes the performance from another perspective from bubble engineering besides the traditional efforts toward catalyst optimization.
DOI:https://doi.org/10.1038/s41467-025-61131-3
