Shao’s team designed a solid polymer electrolyte (SPE) reactor to address the key challenges in the selective electrosynthesis of high-concentration 2,5-furandicarboxylic acid (FDCA) at kilowatt scale and ampere-level current density. This research results were published in “Nature Catalysis” with titled “Selective electrooxidation of 5-hydroxymethylfurfural at pilot scale by engineering a solid polymer electrolyte reactor”.
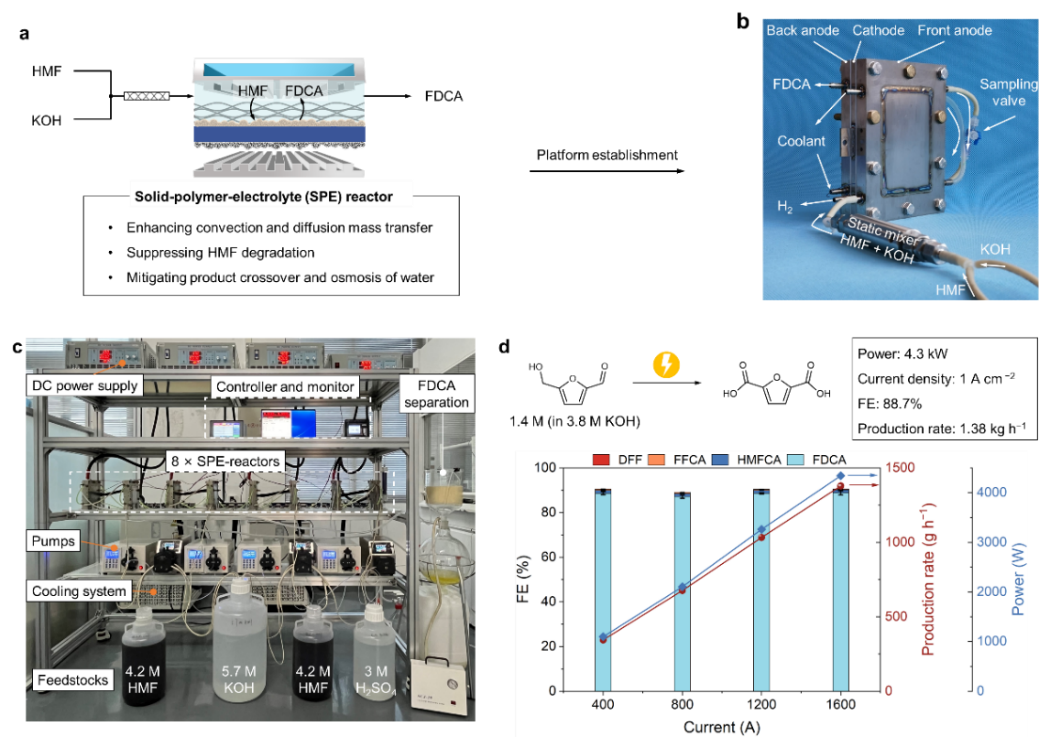
Figure 1. The scheme (a) and photograph (b) of the SPE reactor. c, A photograph of the modular electrochemical platform. d, The catalytic performance at 1.0 A cm−2 of the platform with different numbers of SPE reactors
The research team proposed an engineered SPE-reactor to steer Faradaic and non-Faradaic side reactions, achieving FDCA production at industrially relevant current density (1.5 A cm−2), while maintaining high selectivity (97.0%), Faradaic efficiency (88.2%) and concentration (~1.24 M). The key roles of the reactor include: suppressing Faradaic oxygen evolution by strengthening mass transfer; alleviating non-Faradaic HMF condensation; mitigating undesired crossover of molecules between cathode and anode. The stability of the SPE-reactor was demonstrated in a continuous operation at 0.5 A cm−2 over 140 hours. Furthermore, they demonstrated an engineered SPE-reactor and modular electrochemical platform to enable selective electrooxidation of high-concentration 5-hydroxymethylfurfural (HMF) to FDCA at kW-scale (4.3 kW) and ampere-level current density (1.0 A cm−2). These results represent an important step toward large-scale electrosynthesis of sustainable plastic monomers from biomass.
They also proposed that further research of HMF oxidation should aim at improving the scalability, stability, and economic feasibility. Continuous flow electrochemistry with larger reactors (larger working area, and more modules in serial or in parallel), system integration (feeding, electrolysis, product separation and associated units), and artificial intelligence-assisted automation (electrode fabrication, system monitoring and controlling, self-optimization) may enable acceleration of HMF oxidation industrialization. Advances in catalyst/electrode and ion exchange membrane will increase the stability as well as energy efficiency of the electrolysis process. Further optimization of electrolysis parameters will decrease the capital and operational costs of the process.
Linkage: https://doi.org/10.1038/s41929-025-01374-x
