On November 14, the team led by Prof. Mingfei Shao, in collaboration with Prof. Lei Wang from the National University of Singapore and Prof. Xin Liu from Harbin University of Science and Technology, reported a breakthrough addressing the key challenges of PEM electrolysers—namely their strong dependence on Pt-based catalysts, low cost-effectiveness, and limited scalability. The researchers developed a flame-assisted, rapidly scalable synthesis of a RuP2-encapsulated RuO2 core–shell catalyst, which, when integrated into a practical 2 × 100 cm2 proton-exchange-membrane (PEM) electrolyser, delivers 200 A at a moderate cell voltage of 1.8 V and maintains stability for over 1500 hours. The study, entitled “Scalable ruthenium core–shell hydrogen catalyst for efficient and robust proton-exchange membrane electrolyser,” has been published in Nature Materials. Jinze Li, a 2022 Ph.D. student at Beijing University of Chemical Technology, is the first author, and Beijing University of Chemical Technology is the first-affiliated institution.
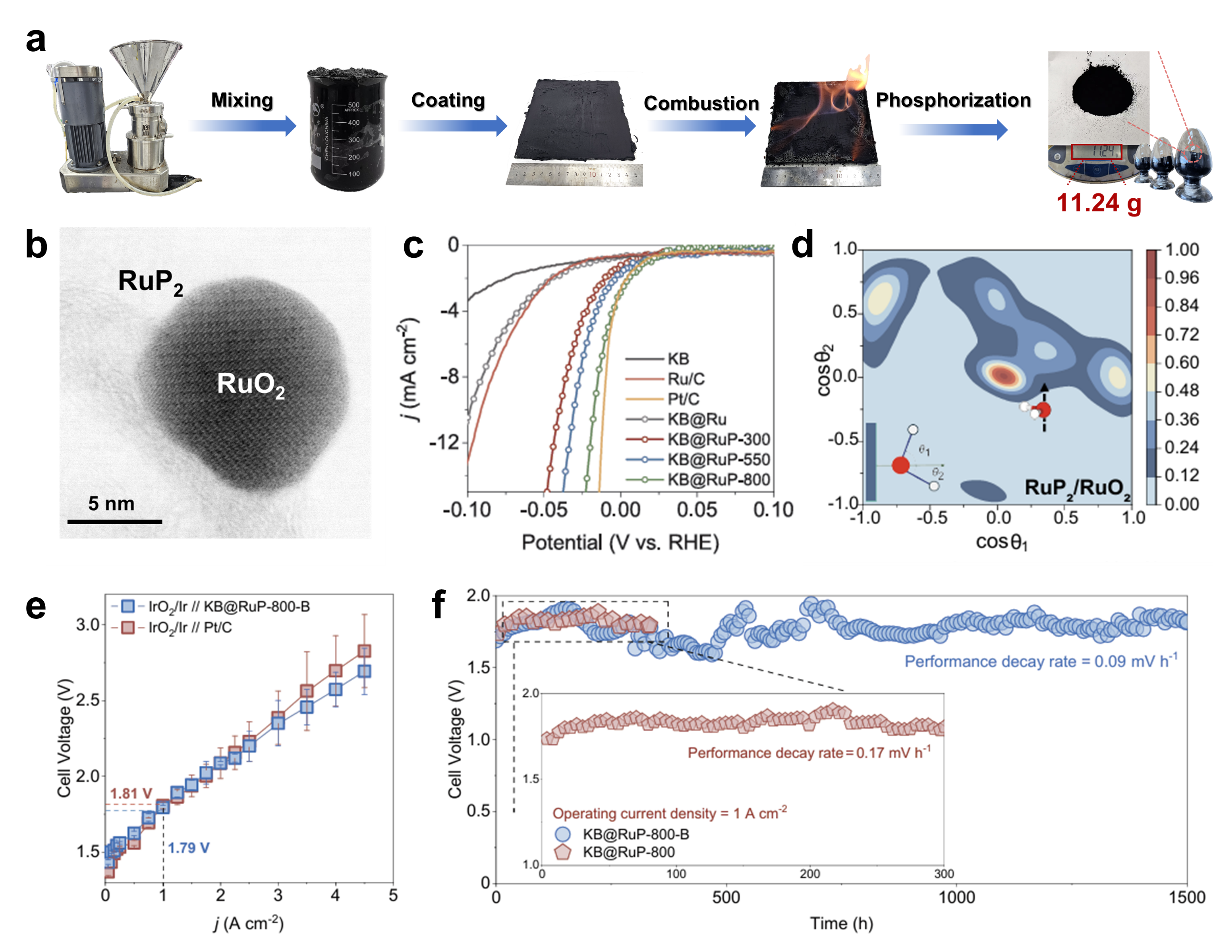
The figure shows the (a) synthesis route of the catalyst, (b) TEM image, (c) electrochemical performance evaluation, (d) interfacial water-molecule orientation distribution, (e) polarization curve in a 100 cm2-scale PEM electrolyser, and (f) stability test.
Green hydrogen produced via water electrolysis powered by clean energy can replace hydrogen derived from fossil fuels, accelerating the future transition to sustainability. Compared to conventional alkaline water electrolyser, proton exchange membrane-based water electrolyser (PEMWE) demonstrates enhanced power density and a compact design, making them more suitable for coupling with intermittent renewable energy sources for cost-effective green hydrogen production. However, PEM electrolysers heavily rely on Pt- and Ir-based electrocatalysts to achieve high power density and industrially relevant lifetimes.
Ru-based materials, costing only 4% of Pt, exhibit comparable H2O binding energies but suffer from rapid oxidation in air, limiting their intrinsic stability in acidic media. Strategies like substrate modification, elemental doping (e.g., P, S, etc.), and forming heterostructures (i.e., core-shell designs) have demonstrated promise in enhancing the durability of Ru-based catalysts. Notably, the high geometric overlap of core-shell structures enables modulation of the catalyst’s electronic structure, which in turn increases its catalytic activity. However, the interfacial electron redistribution and strain effects induced by the core-shell heterostructure are short-range phenomena, emphasizing the importance of controlling the nanoshell thickness to achieve optimal catalytic synergies. Unfortunately, achieving precise control of core-shell structures at nanoscale often requires complex synthetic approaches and stringent conditions, posing substantial challenges to the scaled production of electrocatalysts. Therefore, it is crucial to establish facile and scalable strategies for preparing core-shell catalysts with tunable nanostructures. Such advancements are key to enabling the large-scale fabrication of membrane electrodes (≥100 cm2) for non-Pt hydrogen catalysts, achieving sustained long-term electrolysis delivering a high current output (≥1 A cm2), and ultimately facilitating their practical implementations.
In this work, the authors developed a facile and innovative flame-assisted procedure to prepare a Ru-based catalyst with controllable core-shell structures, comprising a ruthenium oxide (RuO2) core encapsulated by a thin ruthenium diphosphide (RuP2) shell, supported on Ketjenblack (KB) (denote as KB@RuP-800). Detailed kinetic assessments combined with first-principles calculations revealed that the unique thin RuP2-shell/RuO2-core structure, along with the pronounced electronic interaction between these two phases, substantially enhances the intrinsic catalytic activity for HER by modulating the interfacial water structure. As a result, the optimized KB@RuP-800 achieved 10 mA cm–2 at 16 mV vs RHE (Reversible Hydrogen Electrode) in acid, comparable to 20% Pt/C (11 mV). Additionally, thanks to its superior proton conduction that substantially improves charge transfer under operating conditions, the membrane electrode prepared with KB@RuP-800 required only 1.79 V to achieve a current density of 1 A cm–2 in a practical PEM electrolyser, outperforming commercial Pt/C. Owning to the facile synthetic procedure and the mild preparation conditions, the above slurry precursor was produced in bulk at ambient conditions via a colloidal mill, enabling tens-of-grams continuous batches. Remarkably, scaled catalysts in a 2 × 100 cm2 PEM stack sustained 200 A at 1.8 V for over 1,500 h.
Article link: https://doi.org/10.1038/s41563-025-02405-5
